Non-valvular AF is the most prevalent sustained cardiac arrhythmia linked to a high risk of stroke, systemic embolism (SE), heart failure (HF) and all-cause death.1 Without oral anticoagulation, the age-adjusted risk of AF-related stroke increases fivefold.2 For decades, oral anticoagulants (OAC) with vitamin K antagonists (VKA) were the standard therapy for AF-associated stroke and SE, with a 64% relative risk decrease in stroke.3 Because of the narrow therapeutic window of VKAs, it is mandatory for warfarin therapy to stay within adequate therapeutic range as reflected by tests of haemostasis, such as prothrombin time normalised by the international normalised ratio (INR). The time that patients spend in the VKA therapeutic range (TTR) of 65% is rare, even in large randomised trials, while drug compliance and TTR, as expected, are even worse in real life than in randomised controlled trials.4–6
Meta-analysis of all four novel OACs (NOACs) reveals a 19% reduction in the incidence of stroke or SE compared to VKA.7 Left atrial/left atrial appendage (LA/LAA) thrombus is found in 13–19% of AF patients without anticoagulation.8,9 The EMANATE trial reported 7.1% thrombus formation in anticoagulation-naive AF patients and 3.5–17.8% under VKA treatment.10 A recent retrospective cohort study showed that, despite anticoagulation for the recommended 3 weeks before cardioversion, a significant proportion of patients (40%) were found to have LA/LAA thrombus (LAT), especially those on warfarin who had a much higher incidence of this finding compared with on NOACs.11
In patients with nonvalvular AF, LAA thrombosis increases the risk of thromboembolic events.12,13 Implications for long-term stroke and thromboembolism risks due to persistent LAT to long-term anticoagulation are poorly understood. Such refractory LAT may become organised over time and pose a lower embolisation risk than newly generated LAT. This theory is reinforced by the fact that, despite reported rates of LAT detection of up to 3.6% among patients on continuous anticoagulation, recorded rates of thromboembolic events after cardioversion are much lower.14,15 In conclusion, regarding stroke risk, current evidence reveals that both fresh and organised thrombus might be the embolic source. However, because organised thrombus may be challenging to distinguish from the endocardium, a high degree of suspicion might be needed to diagnose an organised thrombus.16
Predictors of Left Atrial Appendage Thrombosis Despite Oral Anticoagulation
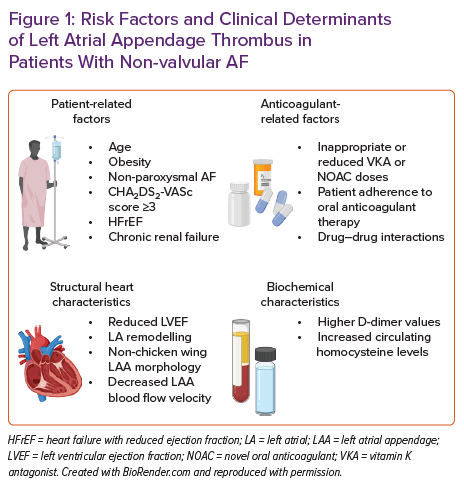
Factors impacting the occurrence of LA or LAA thrombus despite therapeutic anticoagulation with VKA or NOACs among patients with AF are mainly unexplored. In a recent study, Angelini et al. reported that 7.7% of patients with AF referred for catheter ablation or electrical cardioversion had LA/LAA thrombus verified by transoesophageal echocardiography (TOE), despite receiving a guideline-recommended daily dose of NOAC for the purpose of thromboembolic prevention.17 Moreover, 5.1% of all patients had an echocardiographic finding of a dense LA/LAA spontaneous echo contrast, which may precipitate thrombus formation. Finally, they found that this population’s significant predictors of LA/LAA thrombus were CHA2DS2-VASc score >3 and obesity, providing an OR for thrombus presence of 4.54 and 6.01, respectively. This is concordant with previous data from Bertaglia et al., reporting that 3.6% of patients with AF treated with NOAC for at least 3 weeks had LAT visualised by TOE, and all were located in the LAA.18 They also found that this finding was not dependent on NOAC type, while patients with LAA thrombus tended to have a mean CHA2DS2-VASc score of ≥3, thus suggesting that preprocedural TOE in this group might be considered.
Despite anticoagulant therapy, similar findings were reported elsewhere, confirming the association of high CHA2DS2-VASc score and LAT presence and its link to future cerebrovascular events.19,20 However, this relationship is not that simple because even in patients with non-valvular AF and low CHA2DS2-VASc score, elevated plasma homocysteine levels were predictive of LA/LAA thrombus.21 Similarly, in two Polish cohorts enrolling consecutive AF patients of whom the majority or all were receiving oral anticoagulation, the presence of LA/LAA thrombus was 5.7% and 7.5%, respectively, while persistent and permanent AF, renal dysfunction (estimated glomerular filtration rate <56 ml/min/1.73m2), lower mean LAA flow velocity and history of vascular disease were established as solid independent predictors of LA/LAA thrombus formation.22,23
Interestingly, data derived from the retrospective registry of 820 consecutive patients with AF undergoing TOE who were anticoagulated with apixaban for at least 4 weeks before imaging demonstrated that no thrombi were detected in patients with CHA2DS2-VASc score of ≤1.24
Furthermore, LAA morphological architecture and function may differentially impact on thrombogenesis of LA in patients with AF.25 For example, complex LAA morphology characterised by the increased number of LAA lobes was independently associated with LAT, spontaneous echo contrast and stroke in patients with non-valvular AF.26,27 Similarly, non-chicken wing LAA morphology, according to TOE, was associated with an 11.5-fold higher likelihood of LA/LAA thrombosis in patients with non-valvular AF compared to those having a chicken wing LAA formation.28 Decreased LAA flow velocity propagates blood stasis within LAA and this phenomenon occurs in AF; thus, it might independently enhance the risk of thrombogenesis.29,30 Echocardiographic and morphological parameters, such as decreased a-wave rate, increased LA dimensions, atrial sphericity and the degree of atrial fibrosis quantified by late gadolinium enhancement cardiac MRI, were shown to be independently associated with appendage thrombus, thromboembolic events and spontaneous echo contrast in several studies.31–35 The degree of LA dysfunction in non-valvular AF, such as LA emptying fraction <30% in addition to CHA2DS2-VASc score, was a crucial enhancing risk factor for LAT or dense spontaneous contrast in patients with AF.36 Similarly, contrast retention during the LAA occlusion procedure, LAA cauliflower morphology and reduced left ventricular ejection fraction (LVEF) were independently associated with LA/LAA thrombosis.37
Similarly, inappropriately reduced daily dosages of NOACs are likely to enhance the potential for LAA thrombus formation, thus emphasising the need to critically evaluate the pros and cons of NOAC dose reduction in each patient with AF.38 Finally, some drugs concomitantly used with NOACs, such as antiepileptic medications (phenobarbital, phenytoin and carbamazepine), might reduce the therapeutic efficacy of NOACs, thus facilitating the formation of LAT despite guideline-recommended continuous oral anticoagulation.39
Characteristics and Presence of Left Atrial Appendage Thrombus Depending on the LVEF and Presence of Heart Failure
It is recognised that the LA or LAA thrombus can be an essential source of thromboembolism in patients with HF, especially those with a dilated cardiomyopathy phenotype. The anatomical shape of the LAA facilitates haemostasis, which is even more enhanced in cases of poor systolic function and slow flow. Thus, it is a common site for thrombus formation among patients with HF.40 In the subanalysis of the multicentre, prospective, observational LATTEE registry, it was found that the prevalence of LAT was nearly three-fold higher in patients with HF compared to non-HF patients (12.8% versus 4.4%).41 As expected, the LAT presence increased as the systolic dysfunction decreased, meaning that HF with reduced ejection fraction was associated with a significantly 4.1-fold greater likelihood of LAT presence (95% CI [3.13–5.46]) compared to non-HF patients. At the same time, this relationship was insignificant in patients with mildly reduced or preserved systolic function. The multivariable regression analysis within the same study revealed that lower LVEF was an independent predictor of LAT formation, whereas LVEF ≤48% was associated with an increased risk of LAT presence. Of note, this study employed chronic anticoagulation in 88% of patients before TOE; 1.5% were using transient anticoagulation, while only 10% were naive to oral anticoagulation. Age ≥75 years and HF were strongly associated with the presence of LAT among patients with non-valvular AF enrolled in the ENSURE-AF trial.42 Similar findings were validated in a significant meta-analysis pooling 56,660 patients with AF that underwent catheter ablation or electrical cardioversion (ECV). The presence of LAT was 1.3% and 4.9% among those adequately taking OAC, respectively. This study showed that HF was an essential predictor of LAT presence: OR 4.3 among AF patients undergoing ablation and OR 2.8 for those undergoing ECV.43 Interestingly, the OR for LAT was nearly identical for congestive HF patients in the study by Wu et al. (OR 4.4; 95% CI [1.6–12]).15
Novel echocardiographic parameters such as peak LA longitudinal strain (PALS) for LAA thrombus have been recently evaluated among HF patients. Concordantly, in a study that included CHF patients with severely depressed systolic function (LVEF <25%) and sinus rhythm, it was found that LAA thrombus was present in nearly one-third of patients (31.7%), while global PALS was a strong predictor of LAAT (OR 30.4; 95% CI [7.2–128]) for LAAT presence if the measured PALS value was <8%. This study also showed that the tendency for thrombus formation in LAA is significantly enhanced in HF patients with severely depressed systolic function, even in the absence of AF.44
Risk factors, predictors or markers of LA/LAA thrombus are summarised in Figure 1.
Oral Anticoagulant Strategies in Persistent Left Atrial Appendage Thrombus Among Patients Already on Continuous Oral Anticoagulation
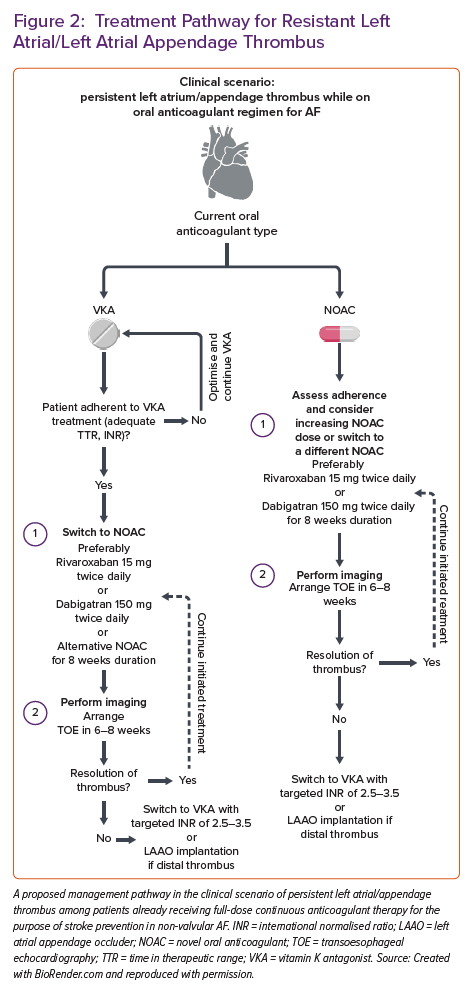
The management strategy in patients with verified LAA thrombus despite therapeutic oral anticoagulation is unclear and mainly based on expert consensus statements or limited case series reports. The recent European Heart Rhythm Association (EHRA) survey conducted among 54 hospital centres showed that in cases of persistent thrombus. In contrast, regarding VKA, most centres would switch VKA in eligible patients to NOAC (42.5% of cases); some would reassess the quality and adherence to VKA in 23.4% of cases. In contrast, 17% would remain on VKA and aim for the higher INR values (2.5–3.5).45 Similarly, about 6.4% of centres would switch from VKA to low-molecular-weight heparin (LMWH). Concerning antiplatelet therapies, the same survey showed that adding antiplatelet agents was infrequent, while none of the centres opted for dual antiplatelet therapy (DAPT). Similarly, the switch to unfractionated heparin among enrolled centres was highly uncommon.
On the other hand, when thrombus was present despite chronic NOAC treatment, the EHRA study showed that the most common strategy was to switch from NOAC (regardless of type) to VKA with a target INR of 2.5–3.5 or to switch to VKA with a target INR of 2–3; these two strategies accounted for about half of all management scenarios. Switch from NOAC to LMWH was used among 6.4–12.8% of participating centres, depending on the NOAC type, with the highest switching rate registered for apixaban and lowest for edoxaban. When NOAC to NOAC substitution was opted for, apixaban and dabigatran were the most commonly tried replacement NOACs. Similarly, the EHRA survey showed that the timing of repeated imaging after the change in OAC remains heterogeneous across centres.45 Nearly half of the centres would repeat imaging 3–4 weeks after the antithrombotic switch, while one-third would repeat imaging after 5–6 weeks. About 11% of centres would opt for delayed imaging arranged >2 months following the antithrombotic switch.
Real-world data might help shed light on the practical use of antithrombotics to resolve refractory LATA thrombus in patients with AF. For example, Faggiano et al. analysed data from 8,888 consecutive patients with AF who underwent TOE in two high-volume clinical centres. Most patients with identified LAA thrombus (3% of the total cohort) were on OAC for at least 3 weeks before index imaging. Their study showed that a VKA for LAA thrombus resolution was prescribed in 52%, NOAC in 27%, and LMWH in 18.5% of patients. Two-thirds of these patients received repeat TOE within a median time of 39 days, while one-third did not receive any follow-up imaging study. Importantly, thrombus resolution was achieved in 67% of all patients who underwent repeated TOE, while no significant difference in efficacy was established between VKA and NOACs.
Kolakowski et al. specifically reported on chronically anticoagulated patients for AF or atrial flutter and still had LAA thrombus detected by the TOE.46 They showed that nearly 52% of patients had LAA thrombus dissolution regardless of the number of treatment cycles employed. In contrast, any change in treatment (switch to a different OAC) was associated with increased odds of success. However, it is unclear whether any particular treatment strategy is more effective than the other. Additionally, the authors showed that several anticoagulation treatment cycles and the left atrium area were adversely related to thrombus resolution. Nelles et al. performed a similar study with their retrospective single-centre registry analysis, including 78 patients with AF. In that patient cohort, a large proportion of participants were diagnosed with solid LAT despite being treated with NOAC (45% of patients) or VKA (41% of patients).47 Their data show how thrombus resolution was achieved in almost half the enrolled patients during the mean follow-up time of 1 year, without a significant difference in efficacy between NOACs and VKAs. However, among those patients that responded to therapy with visualised thrombus resolution, there was a significantly shorter mean time to achieve that with NOACs versus VKA (81 versus 129 days; p=0.03).
Harada et al. previously showed how administering 300 mg of dabigatran (150 mg twice daily) in patients with LAAT and AF while on continuous NOAC therapy was effective in achieving thrombus resolution. However, this finding was obtained in a small sample size, and previous adherence to NOACs was not carefully evaluated.48 Similar results were obtained in a small-sized study by Yilmaz et al., including 17 patients with AF and LAA thrombus who also completed baseline and follow-up TOE examinations after initiating or switching their anticoagulation regimen.49 Patients in their study were treated with 300 mg dabigatran daily. Thrombus resolution was achieved in 87% of patients (7/8, all paroxysmal or persistent AF). At the same time, it was ineffective in only one patient with long-standing continuous AF. In another report, two patients with LAT resistant to rivaroxaban had thrombus resolution after starting dabigatran.50 Dabigatran is the only OAC that serves as a direct thrombin inhibitor and a prodrug. In contrast, the others (rivaroxaban, apixaban and edoxaban) act as factor Xa inhibitors in their active forms, thus reflecting different mechanisms of action. They concluded that dabigatran given twice daily was more efficient than a factor Xa inhibitor given once daily at dissolving existing thrombi and preventing the creation of new ones.50 The RIVA-TWICE prospective open-label study declared that when standard rivaroxaban therapy fails, rivaroxaban 15 mg twice daily appears as a safe therapeutic option and may dissolve LAA thrombus, with a resolution rate of LA/LAA thrombosis of 46.7%.51
A recent systematic review and meta-analysis comparing the use of NOAC versus warfarin for the treatment of LA thrombosis in patients with non-valvular AF showed that NOAC use was associated with a 2.2-fold increased probability of LAT resolution and this was not offset with higher risks of bleeding or stroke/transient ischaemic attack (TIA).52 However, cautious interpretation of this analysis is advised since previous/current anticoagulation varied greatly across included trials. Some trials did not report previous anticoagulant exposure; some had all patients covered by NOACs or VKAs, while some enrolled patients were not previously treated with OACs.
The formal approach and management strategy are laid out in the recent EHRA 2021 practical guide on using NOACs in patients with AF.53 This document recommends that the management be individually tailored to each patient with AF with the persistent thrombus regardless of good adherence to NOAC treatment. Some general principles to consider are provided in this document – patients might be switched to a NOAC with a different mechanism of action (for example, switching from factor Xa inhibitor to direct thrombin inhibitor or vice versa) or to VKA therapy with a customised INR target. Similarly, non-pharmacological alternative strategies such as LAA closure with dedicated devices might be considered in particular clinical scenarios. However, the authors clearly state the lack of prospective evidence in this setting.
Therefore, it becomes clear that all management decisions should be carefully balanced by estimating each individual patient’s bleeding and thrombotic risks. Only a few options have been available regarding the results of LAA closure in patients with AF and LAA thrombus. A recent review that comprised 35% of patients whose LAA thrombosis was persistent and distally situated demonstrated that LAA occlusion (LAAO) was possible in these individuals.54 The WATCHMAN device (Boston Scientific) requires the delivery sheath to be progressed into the LAA until its marker aligns with the LAA’s ostial plane, which may increase the risk of distal contact and embolisation.55 In this patient subgroup, the lobe and disc devices might be a better option for LAA closure.
Herein, we propose a management scheme to patients with persistent LA/LAA thrombus despite full-dose anticoagulation for non-valvular AF (Figure 2).
Adding Antiplatelet to Anticoagulation Drugs for the Pharmacological Resolution of Persistent Left Atrial Appendage Thrombus
To date, no randomised data or studies show the superiority or increased effectiveness of adding an antiplatelet agent to the existing or switched anticoagulant regimen for this indication. In a retrospective work by Kolakowski et al., it was suggested that keeping the same anticoagulant medication but adding an antiplatelet agent was associated with a numerically greater efficacy compared to several other strategies for LAA thrombus resolution (e.g. switch to an anticoagulant with a different mechanism, switch to an anticoagulant with a similar mechanism of action, switch to another anticoagulant with an added antiplatelet agent, adding a second anticoagulant drug or deliberate no change in treatment).46 However, combining an OAC and antiplatelet failed to show a statistical advantage in efficacy over any other antithrombotic regimen. It can be concluded that the role of antiplatelet addition for this indication is highly limited and currently not supported by the evidence except in cases in which a patient has another indication, such as concomitant coronary artery disease.
Continuous Oral Anticoagulant Regimen Following Left Atrial Appendage Occlusion Device Implantation
While oral anticoagulation therapy is effective in mitigating thromboembolic risks in non-valvular AF, for some patients bleeding risks and nonadherence to therapy present important barriers in effective anticoagulation. For these patients, surgical and percutaneous LAAO devices are important non-pharmacological strategies to overcome the challenges of anticoagulant pharmacotherapy.56 LAAO is also a feasible and safe therapeutic option for those patients that suffered a cerebrovascular event despite being on adequate anticoagulant treatment.57
The recent meta-analysis of observational data showed no difference in stroke, major bleeding, device-related thrombosis, and all-cause mortality rates in patients receiving antiplatelet versus anticoagulant agents following LAAO.58
However, whether patients after LAAO should still receive anticoagulants and, if yes, for how long and at what dose remains an open question in clinical practice.59 The results of the real-world prospective study in which 41% of patients did not receive OAC while 59% received OAC after LAAO with the LARIAT device (SentreHEART Inc) showed that there was no difference between the two groups in relevant outcomes such as rates of ischaemic stroke/TIA, thromboembolic events, bleeding, life-threatening, disabling or significant events, and annual mortality rate.60 Cepas-Guillen et al. recently conducted a study in which a low-dose strategy with apixaban (2.5 mg twice daily) was tested against single antiplatelet therapy (SAPT; low-dose aspirin 100 mg once daily) and DAPT (aspirin 100 mg + clopidogrel 75 mg once daily) in patients with non-valvular AF who underwent LAAO.61 The authors concluded that a strategy with low-dose apixaban following LAAO might be a feasible and effective alternative to DAPT and SAPT concerning combined efficacy and safety endpoints. However, this study was not randomised and enrolled a limited number of patients. In the ADRIFT trial, strategies of two doses of rivaroxaban were compared versus DAPT consisting of 75 mg aspirin and 75 mg clopidogrel in patients implanted with Amplatzer Amulet (Abbott) and WATCHMAN devices for LAAO.62 This study showed that the circulating levels of prothrombin fragments 1 and 2 reflecting thrombin generation following the LAOO procedure were higher among patients treated with DAPT than 10 or 15 mg rivaroxaban. However, it remains unclear whether this effect can reduce adverse post-procedural events such as device-related thrombosis or other thromboembolic events. In line with this, Tjoe et al. showed, in a retrospective analysis of 213 patients, that use of DOAC with or without aspirin had similar safety and efficacy profile post-WATCHMAN device implantation when compared to warfarin and aspirin use.63
Furthermore, robust nationwide data on oral anticoagulation following LAAO became recently available from the LAAO Registry of the National Cardiovascular Data Registry that enrolled patients implanted with the WATCHMAN device in the US.64 This extensive analysis of 31,994 patients who underwent successful LAAO showed that the most significant deviations from implantation protocol were observed in post-discharge antithrombotic medications. This analysis showed that the post-implantation discharge on warfarin or DOAC, compared to DOAC + aspirin or DAPT alone, was associated with a significant reduction in the composite endpoint of adverse outcomes.
Conclusion
Taken together, it seems that in case of LAA thrombus presence despite chronic anticoagulation treatment, most centres would practice switching to another anticoagulant drug with a different mechanism of action. In contrast, repeated imaging for LAA thrombus would be performed within 3–6 weeks in over 80% of cases. It is also evident that several essential questions in the scenario of LAA thrombus – despite apparently adherent chronic OAC treatment – remain unanswered. These are which anticoagulation drug should be selected in these cases, for how long treatment should be initiated, which dosing regimen should be selected and when should the follow-up imaging be arranged. As previously elaborated, relevant meta-analysis suggests increased efficacy with NOACs than warfarin, and there is limited clinical evidence that 300 mg of dabigatran might be particularly effective. However, these observations need to be confirmed in a prospective randomised fashion. In summary, it becomes evident that the optimal choice, dosing and duration of antithrombotic and anticoagulation treatment following LAAO is unclear and that high-quality large randomised trials adequately powered for relevant clinical outcomes are warranted. The role of continuous anticoagulant use following LAAO implantation would need to be prospectively validated by such studies.