Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, is associated with increased risk of stroke and heart failure and is a significant global health challenge.1 Pharmacological treatments to restore sinus rhythm in patients with AF are associated with a considerable relapse rate,2,3 whereas nonpharmacological interventions, such as catheter ablation procedures, which isolate the pulmonary veins (PV) from the left atrium and prevent AF initiation, are associated with long-term (≥3 years after ablation) success rates of up to 80 % with multiple procedures.4 Rates of ablation procedures have been steadily increasing over the last decade,5 but in order for catheter ablation to become first-line treatment for AF patients, there is a need for higher and reproducible single-procedure success rates with a substantially reduced procedure time. In order to achieve this, durable transmural and contiguous radiofrequency (RF) lesions are required in concert with standardisation of the ablation strategy. An important part of the challenge is determining whether sufficient RF energy has been delivered to the appropriate local tissue site: the most common cause of failure of catheter ablation or arrhythmia recurrence is electrical reconnection of the PVs following suboptimal energy delivery,6 while excessive energy can result in overheating, local injury, perforation, fistulae, steam pops and char.7,8 Contact force (CF) between the ablation catheter tip and the target tissue is a key determinant of the locally delivered RF energy. Although applicable to other arrhythmia substrates as well, this review will mainly consider the impact of CF on procedural success of AF ablation.
Effect of Contact Force on Lesion Size
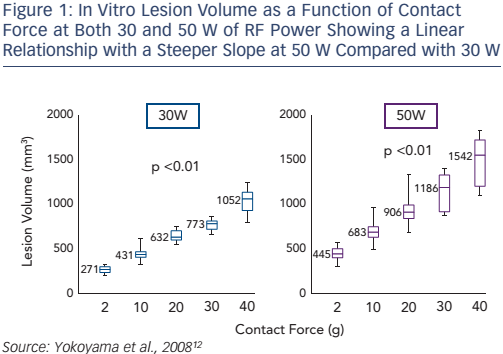
A number of in vitro studies have shown that CF affects lesion size in RF catheter ablation.9,10 We described the first results of in vitro ablation with an optical fibre based real-time CF sensor equipped RF irrigation catheter showing that increased CF was associated with larger and deeper lesions despite fixed RF power.11 In 2008, a thigh muscle study confirmed that the underlying mechanism was related to increased tissue heating (see Figure 1): at high CF there was an increased incidence of steam pop, and thrombus.12
A 2010 in vitro study found that integrating CF information over time (for the first 40–60 seconds) provided a direct correlation with lesion size, again in the setting of stable fixed RF powers.13
Effect of Contact Force on Clinical Outcomes
Technologies are now clinically available that allow the direct measurement of CF between the catheter tip and the target myocardium in real-time, and two CF sensing catheters have received approval by the US Food and Drug Administration for use in AF ablation. The first catheter approved was the ThermoCool® SmartTouch (Biosense Webster) irrigated tip ablation catheter (February 2014) followed by the TactiCath Quartz (St. Jude Medical) CF ablation catheter (October 2014). A growing body of clinical data, both observational and larger studies, supports their use.
The first published study of the use of CF ablation catheters in humans was the Touch+™ for Catheter Ablation (TOCCATA) study (n=77), which demonstrated that catheter ablation using real-time CF technology was safe for the treatment of supraventricular tachycardia and AF.14 In this study, a mean CF of 20 g or more correlated with an 80 % probability of AF freedom at 12-month follow-up.
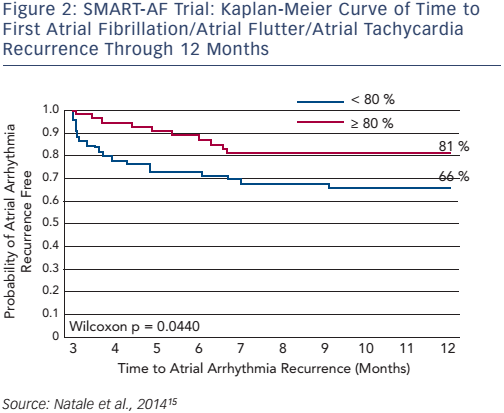
SMART-AF (Prospective Safety Assessment of the ThermoCool® SmartTouch® SF Family of Contact Force Sensing Catheters for the Radiofrequency Ablation Treatment of Drug Refractory Symptomatic Paroxysmal Atrial Fibrillation) was a prospective, multicentre, nonrandomised study (n=172) that assessed the safety and effectiveness of the ThermoCool SmartTouch catheter in the treatment of drug-refractory symptomatic paroxysmal AF (PAF). The 12-month freedom from AF/atrial flutter/atrial tachycardia recurrence was 72.5 %. When the CF was stable within an investigator-selected range ≥80 % of time, the success rate increased to 88 % (≥80 % of time: n=32; <80 % of time: n=73; see Figure 2).15
TOCCASTAR (Ablation Catheter Study for Atrial Fibrillation) was a prospective randomised noninferiority study that compared the TactiCath catheter with the Navistar Thermocool catheter (Biosense Webster) in patients undergoing catheter ablation of paroxysmal AF. The 12-month freedom from AF/atrial flutter/atrial tachycardia recurrence was 68 %. A prespecified analysis of TOCCASTAR investigated the impact of CF in the TactiCath cohort. Of the 145 patients treated with the TactiCath catheter, 57 % received an optimal amount of contact (90 % lesions delivered at 10–40 g of CF) and experienced a chronic treatment success rate of 85.5 % versus 72.6 % for the suboptimal CF group (p=0.043).16 These results highlight the need to standardise operating procedures so as to routinely achieve optimal CF.
An analysis of these and other data shows that successful PV isolation (PVI) is a function of transmurality, i.e. lesions extending through the entire thickness of the atrial wall as well as lesion continuity. Transmurality is affected by wall thickness, oedema and tissue composition, while contiguity, requiring the 3D localisation of the catheter, is affected by the lesion shape, interlesion gap and compensating for or evaluating electrode sliding. These are currently difficult parameters to measure. In this context, a varying optimal CF value has been suggested in different studies with, for example, Providencia et al. finding a mean CF <22 g indicative of poorer 12 month outcomes,17 whereas Stabile et al. found no difference in mean CF (13 ± 3.4 g versus 12 ± 4 g; p=0.32) between patients with and without arrhythmia recurrences.18 Whether these data indicate differences in tissue characteristics, RF power delivery, catheter tip stability or combinations of the above is unclear and prospective investigation using indices combining CF and other parameters (see below) may provide better results and greater safety.
As increasing CF leads to higher tissue temperatures, excessive CF can lead to collateral damage related complications. Although most studies have shown similar or even fewer complications associated with the use of real-time CF sensing catheters compared with non-CF sensing catheters, these studies have all been relatively underpowered to detect small differences in complications. Excessive CF has been associated with perforation14 and tamponade,15 and perhaps atrioesophageal fistula, 19 thus underlining the importance of optimising CF.
Optimising Contact Force
The CF shows significant variability in everyday use; one reason for this is the cardiac rhythm during catheter ablation. In one study (n=20), the main reasons for CF variability were identified as systolo-diastolic heart movement (29 %) and respiration (27 %), with the rest presumably due to electrode sliding unrelated to cardio-respiratory movement.20 Catheter contact stability is important and can be considered to be a combination of both temporal contact stability (which refers to degree of contact variation over time) and spatial contact stability (which refers to stability in terms of spatial localisation with respect to the endocardium).21 Realtime CF monitoring is crucial in achieving temporal stability, and by implication, transmurality, whereas accurate 3D localisation with respect to the endocardium is necessary for achieving spatial contact stability (as opposed to a fixed extracardiac/extrathoracic reference, which is currently the state of the art), which is crucial for individual lesion localisation and therefore multi-lesion contiguity.21
There are, however, other parameters beyond catheter tissue contact which are important in determining RF lesion size and contiguity.
A significant consideration for obtaining complete lesions is the wall thickness of the pulmonary vein–left atrial (PV–LA) junction. In a recent study,18 AF patients underwent wide area circumferential ablation with a target CF >10 g at a power setting of 25–30 W for 30 seconds. Of the 974 ablation points, 72 were located at dormant conduction sites and were strongly associated with thickened PV-LA junction walls (1.02 ± 0.23 versus 0.86 ± 0.26 mm; p<0.0001) and decreased impedance fall (13.3 ± 6.4 versus 14.9 ± 7.1 ohm; p=0.0498) but not with electrogram-based information or CF. Multivariate analysis identified the thickened PV–LA junction wall as the strongest predictor of dormant conduction.22
Lesion contiguity and density are also important. A study (n=40) found that the majority of pulmonary vein reconnections (PVR), an important cause of AF recurrence after ablation, could be attributed to inadequate CF (<10 g) or long (>5 mm) interlesion distances.23 Another study (n=49) found that, in order to prevent PVR, it was necessary to complete PVI with circumferential lines without touch-up ablation, and also to create a sufficient lesion density (anterior left pulmonary vein [LPV] 1.97/cm and posterior LPV 2.01/cm) on the lines with an adequate CF (mean 14.2 g).24 As alluded to earlier, modern 3D mapping systems are important aids in providing information about spatial stability although they take into account only the catheter tip position under the assumption of static endocardium. Despite this limitation, automatic visual annotation algorithms (e.g. Visitag from Biosense, Automark from St Jude) have become popular, allowing user programmable settings of spatial stability (in time and space) during RF delivery and, combined with lesion prediction algorithms, can provide useful information about weak spots in circumferential PV isolating lesions.25
Force Sensing with RF Ablation versus Cryo-ablation
A multicentre European study compared PVI by CF-guided RF with second-generation cryoballoon (CB). The procedure time was lower for the CB group than for the CF group (109.6 ± 40 versus 122.5 ± 40.7 min; p=0.003), but fluoroscopy duration and X-ray exposure showed no statistical differences (p=0.1 and p=0.22, respectively). In addition, the overall complication rate was similar in the CB and CF groups (7.3 % versus 7.1 %; p=0.93).26 In another study comparing the two techniques, 56 consecutive patients underwent a repeat ablation due to recurrent atrial tachyarrhythmias after the index PVI achieved with CF ablation (30 patients) or CB ablation (26 patients). The percentage of reconnected PVs was significantly lower following CB procedures than with CF ablation (36.1 % PVs in the CF catheter ablation group, versus 20.4 % PVs in the secondgeneration CB ablation group; p=0.01).27 However, the mean CF perreconnected vein was lower compared with the persistently isolated PVs (10.9 ± 2.7 versus 18.6 ± 3.1 g; p<0.001), indicating the potential of further reduction in PV recurrence by achieving higher CFs. The same cannot be said of CB PVI since despite the finding that late PV reconnection was associated with warmer nadir temperature (-48.9 ± 5.1 versus -51.2 ± 4.7 °C; p=0.05), no specific measures are available to address this poorer cooling, and certainly none targeted to the specific area of conduction recovery.
A study performed in seven UK centres randomised patients to ablation with (n=58) or without (n=59) CF data available to the operator. PVR was assessed with adenosine at 60 minutes and all the connections were re-isolated. In the group for which CF data was available, a reduction in acute PV reconnection rates was seen (22 % versus 32 %; p=0.03) but this did not translate to a significant difference in 1-year success rates (defined as off antiarrhythmic drugs) between the two groups (49 % versus 52 %; p=0.9).28 There may be a number of reasons for this. Firstly, the dormant PV conduction was ablated at 60 minutes, thus equalising the long-term outcomes between the two groups while the analysis was only performed with the initial ablation data. PV reconnection was assessed as dichotomic rather than number of gaps or segments with gaps and it is conceivable that non-CF guided PVs would have demonstrated more or wider gaps. In addition, the CF range was large, and it is unclear whether stable CF was assessed during ablation.
Lesion Assessment During Catheter Ablation
It is evident that the ability to assess ablation lesion ‘quality’ during PVI would help in achieving better complete and stable lesions. Lesion assessment tools can help predict and localise electrical weak points, including actual or potential gaps with the potential of correcting these weak points with additional optimised RF energy delivery. Current diagnostic catheters allow the assessment of electrically active and conducting gaps, but provide no feedback on changes from ablation in the myocardial tissue itself and in particular lack information on whether the electrical changes – and by implication, tissue changes – are reversible or irreversible. Ideally, lesion assessment should provide information about dimensions including depth, diameter and also shape of the irreversibly necrosed tissue as well as of the zone of reversible thermal damage. Additional information of potential utility could include peak tissue and interface temperatures as well as electrogram or activation pattern changes. Direct real-time interrogation of tissue temperature could provide much of the above information but is currently not clinically achievable.
Thermal ablation modelling has therefore been proposed as a means of monitoring and visualisation of the tissue response during RF ablation. In a preliminary study evaluating a comprehensive heat transfer model incorporating both conductive and resistive heat transfer as well as perfusion-mediated heat dissipation (convection), four fibre optic probes were inserted into the tissue sample at 2.5 mm and 5 mm radially away from the electrode and at 3 mm depth. Temperature measurements were simultaneously recorded from all probes during the 60-second ablation procedure at 90 °C. There was less than 5 °C difference between the model-predicted and measured temperature throughout the duration of the ablation attesting to the fidelity of thermal modelling incorporating all elements of the heat transfer equation.29
In the clinical context, combining RF energy, duration and contact parameters can allow reasonably accurate but probabilistic prediction of lesion size and therefore potential weak points within a chain of multiple contiguously delivered lesions. Force–Time Integral (FTI), the area under the real-time CF curve, can be considered the first generation lesion prediction assessment index. It was validated first in in vitro studies and against clinically available indirect parameters such as adenosine provoked dormant conduction as well as by direct restudy 3 months after initial PVI. The Efficas I study validated a minimum FTI of 400 gs as providing an 95 % likelihood of a gap-free encirclement.30 Although FTI is commonly used as a marker of ablation lesion quality it does not incorporate power, (which, in most laboratories, is either stable or varies only little) and depicts a linear, instead of the actual non-linear, relationship of lesion growth with time. In an attempt to improve the prediction of PVI success beyond that shown with FTI.13,16,30–33 RF power was incorporated into a non-linear formula, providing the so-called lesion index (LSI). This index was derived from a database of large animal (porcine and canine) ventricular lesions, which could be accurately evaluated post mortem.33
Similarly, the ablation index (AI), based on integrating RF power, duration and CF, has also been proposed as a tool to assess the quality of ablation lesions. It too has been derived from a ventricular large animal RF lesion database. In a recent study,34 40 patients with paroxysmal AF underwent CF-guided PVI and the mean AI identified for each PV segment. The default power was 30 W: 25–30 W for the posterior wall and 30–35 W elsewhere. The CF ranged from 5–40 g and the RF duration from 20–40 seconds. At 2 months follow-up, late PVR was observed in 11 % of segments in 62 % of patients. The minimum AI (as well as FTI) was significantly lower in reconnected segments (308 versus 373; p<0.0001) compared with non-reconnected segments. Higher AI values were documented for anterior/roof segments than for posterior/inferior segments to prevent reconnection.34 This finding may reflect both local wall thickness differences as well as differences in catheter stability.
Another small study including 42 patients undergoing PVI used an index (ALCI) derived from depth and contiguity parameters (including CF parameters: FTI, AI and interlesion distance) to predict the weakest spot in the ablation-isolation lesion and found that minimum ALCI, significantly lower in segments with PVR (74 % versus 104 %; p<0.001), was the most accurate in identifying sites of acute as well as late (at repeat ablation) PV reconduction.25 PVR sites were also characterised by a lower minimum AI (367 versus 408 arbitrary unit [au]), and a higher maximum ILD (inter-lesion distance; 6.8 versus 5.5 mm).
Future Directions
These data highlight the need for precise and accurate lesion prediction, incorporating not only CF, power and time, but also tissue thickness, high-resolution electrograms, catheter tip orientation and stability with respect to the endocardium to ensure the creation of durable optimal ablation lesions.
Other Arrhythmia Substrates
The largest body of data on CF sensing is related to catheter ablation of AF. However other substrates including (but not limited to) typical cavotricuspid isthmus dependent flutter as well as both epicardial and endocardial ventricular arrhythmia substrates have been investigated. In typical flutter, low CF was implicated in longer time to achieve conduction block and increased risk of acute reconnection.35 In the ventricles, higher CFs led to larger lesions but intriguingly also provided greater electrogram information such as higher amplitudes and more frequent late potentials.36
Conclusion
RF catheter ablation of AF has been considered a challenging procedure, but the availability of CF sensing technology allows real-time feedback of exactly how much CF is applied at any given moment, minimising the likelihood of applying too much as well of too little force. A growing body of evidence supports the use of CF sensing technology. Real-time CF information may help to create more predictable and reliable lesions and potentially improve both the safety and efficacy of RF ablation. Despite the absence of prospective randomised clinical studies with longer follow up, RF ablation guided by real-time CF sensing has become the dominant ablation modality, particularly for catheter ablation of AF.37–39 If however, the better acute outcomes with reduction in acute electrical PV re-connection shown in medium term studies,40 is borne out in studies with appropriate longer-term follow-up, then CF sensing technology has the potential to become the recommended standard of care for all patients undergoing AF catheter ablation.







