Abstract
Ultra-low-density fiberboards (ULDFs) were prepared by a liquid frothing technique using plant fibers as the matrix and Si-Al compounds as the filler to be used as a versatile bio-based composite. Si-Al compounds played an important role in the fire properties of ULDFs. Fire intensity and the amount of volatiles were significantly restrained because of the Si-Al compounds. To determine the combustion mechanism of ULDFs treated by Si-Al compounds, the microstructure of burned specimens was tested by chemical analysis, X-ray diffractometer (XRD), and infrared spectrometer (IR). According to the results from gas chromatography, glucose, xylose, and mannose disappeared in the bottom ashes. After combustion, the XRD profiles of the two ashes became weaker and broader; the sharpest peaks at 18.6° (2q) that represented Si-Al compounds remained; the obvious peaks at 22° (2q) from cellulose were gone. The results from IR suggested the characteristic functional groups OH, CH, and C=O from carbohydrate also disappeared, and absorbance at 1200 to 400 cm-1, which attributed to the vibration of Si-O, Al-O, and Si-O-Si bonds, increased. In conclusion, fibers are almost completely pyrolyzed at 780 °C. The crystalline structure of Si-Al compounds is rearranged and more amorphous silicon oxide and aluminum oxide are generated.
Download PDF
Full Article
Microstructure of Burned Ultra-Low-Density Fiberboards using Plant Fiber as the Matrix and Si-Al compounds as the Filler
Min Niu,a,b Xiaodong (Alice) Wang,b Olle Hagman,b Olov Karlsson,b and Yongqun Xie a,*
Ultra-low-density fiberboards (ULDFs) were prepared by a liquid frothing technique using plant fibers as the matrix and Si-Al compounds as the filler to be used as a versatile bio-based composite. Si-Al compounds played an important role in the fire properties of ULDFs. Fire intensity and the amount of volatiles were significantly restrained because of the Si-Al compounds. To determine the combustion mechanism of ULDFs treated by Si-Al compounds, the microstructure of burned specimens was tested by chemical analysis, X-ray diffractometer (XRD), and infrared spectrometer (IR). According to the results from gas chromatography, glucose, xylose, and mannose disappeared in the bottom ashes. After combustion, the XRD profiles of the two ashes became weaker and broader; the sharpest peaks at 18.6o (2) that represented Si-Al compounds remained; the obvious peaks at 22o (2) from cellulose were gone. The results from IR suggested the characteristic functional groups OH, CH, and C=O from carbohydrate also disappeared, and absorbance at 1200 to 400 cm-1, which attributed to the vibration of Si-O, Al-O, and Si-O-Si bonds, increased. In conclusion, fibers are almost completely pyrolyzed at 780 °C. The crystalline structure of Si-Al compounds is rearranged and more amorphous silicon oxide and aluminum oxide are generated.
Keywords: Ultra-low density fiberboards (ULDFs); Si-Al compounds; Combustion; Microstructure
Contact information: a: Department of Wood Science and Technology, Fujian Agriculture and Forestry University, 350002, Fuzhou, Fujian; b: Division of Wood Products Engineering, Luleå University of Technology, 93162, Forskargatan, Skellefteå, Sweden; *Corresponding author: fafuxieyq@aliyun.com
INTRODUCTION
To improve the energy efficiency of houses built in cold regions, extensive insulation material is applied during construction of the house. Glass fiber (Isover Co.) and rock wool (RockWool Co.) are the most popular mineral-based insulation materials in the current market of building materials. Because it can offer similar properties, bio-based insulation material as an alternative product has a potentially huge development opportunity because of the abundance of raw materials, good physical and mechanical properties, and favorable effects on the environment.
Many efforts have focused on the manufacturing method and properties of bio-based insulation materials during the past ten years. For example, this working group has applied a liquid frothing approach to develop this type of material. Through this work, fiberboards with an ultra-low density of 10 to 90 kg/m3, thickness swelling of 0.57% after 24 h of water immersion, low thermal conductivity of 0.035 W/mK, high sound reduction coefficient of 0.67, and good fire resistance and mechanical properties have been obtained (Xie et al. 2004, 2008a, b, 2011; Niu et al. 2014; Chen et al. 2015).
Kawai’s working group (Kawai et al. 1998; Xu et al. 2004) studied the manufacture and properties of particleboard using kenaf core (Hibiscus cannabinus L.) as a raw material and of fiberboard using wood fiber as a raw material with density ranges from 50 to 500 kg/m3 formed by steam injection. Frenette et al. (1996) studied cellulosic fiber insulation material using short (1 to 4 mm) cellulosic fibers as filler and bonding synthetic fibers as the matrix. Cellulosic fiber is usually composed of 5 to 8% of synthetic fibers in weight. HempFlax Co. (hempflax.com) and Dieffenbacher Co. (www.dieffenbacher.de) produce natural insulation fiberboard using a traditional fiberboard production line. Also, Cervin et al. (2013) studied light-weight nanofibrillated cellulose foam with a porosity of 98% and a density of 30 kg/m3.
However, the two most important parameters for the evaluation of bio-based building insulation materials are thermal conductivity and fire resistance. Therefore, the addition of fire retardants is essential to change the two parameters. Compounds such as halogens, silicate, boron, and FRW (composed of guanylurea phosphate, boric acid, and a small amount of additives), which are typically used to improve the fire resistance of wood, were employed as fire retardants for ultra-low-density fiberboards (ULDFs) in Liu’s study (2013).
Si-Al compounds with a pH value of approximately 3.8, in the form of an aqueous mixture of sodium silicate and aluminum sulfate, were used in subsequent studies because of the improved high-temperature resistance and cooperation with other fire retardants (Niu et al. 2014). The inorganic substances were relatively evenly dispersed on the surface of fibers and filled in the spaces between the fibers. This significantly decreased fire intensity and the amounts of volatiles released in the process of combustion. In addition, silicon and aluminum compounds have also been applied to modify solid wood and obtain organic-inorganic composites with desirable fire properties (Wang et al. 2000; Zhou et al. 2000).
The microstructure of burned wood has been reported (Zicherman and Williamson 1981); however, researchers have not yet reported the structural changes of burned ULDFs with Si-Al compounds. The aim of this study is to clarify the main mechanism behind fire resistance of ULDF with Si-Al compounds by parameters and experiments.
EXPERIMENTAL
Materials
Recycled kraft pulp (spruce-pine-fir, Tembec Inc., Canada) and waste newspaper (collected in China) were used as raw materials in the preparation of ULDFs. An aqueous solution of sodium silicate and aluminum sulfate was applied as a reinforcing agent to improve the fire properties of the materials; the applied dosages were 500 and 900 mL. Fibers, Si-Al compounds, glue, a water-proof agent, and a surfactant were blended together to get a foaming solution with an increasing volume. The detailed manufacturing process was described in a previous study (Niu et al. 2014).
The ULDFs were burned in a cone calorimeter (FTT Co., England) at 780 oC, in accordance with ISO 5660-1/2002, to obtain fly ash (upper ash caused by complete combustion) and bottom ash caused by incomplete combustion. The two types of ash, along with unburned ULDFs, were used as the specimens for further testing.
Methods
Carbohydrate analysis
Carbohydrates in unburned ULDFs and bottom ash were analyzed according to the methods of Theander and Westerlund (1986) and Karlsson et al. (2012). The specimens (60 to 61 mg) were dissolved and dispersed in a screw-capped glass bottle (20 mL) using sulfuric acid (12 M, 0.5 mL) with magnetic stirring (0.5 h) and vacuum-pumping (1.5 h). The samples were diluted using distilled water (8.4 mL) and kept capped in hot steam from boiling water in a pressure-cooker for 2 h.
After cooling to ambient temperature, an internal standard solution of 2-deoxygalactose (5 g/L, 1 mL) was added to clearly separate the mixture into two portions by shaking. The supernatants (1 mL) were transferred into a small glass tube (5 mL), and the pH value was adjusted to 7 using ammonia hydroxide (12 M, 100-200 μL), then reduced in a temperature-controlled water bath at 40 oC for 1 h with aqueous ammonium hydroxide (3 M,100 μL) solution containing potassium borohydride (150 mg).
The pH value of the solution was adjusted to 7 again by addition of glacial acetic acid (100 to 200 μL). The supernatant (500 μL) was reacted with 1-methylimidazole (0.5 mL) and acetic anhydride (5 mL) for 10 min in a screw-capped tube (30 mL) with gentle shaking.
Absolute ethanol (1.0 mL), distilled water (5 mL), and potassium hydroxide (7.5 M, 5 mL) were very slowly and orderly added to the tube at intervals of a few minutes. The tubes were vibromixed and left for 10 min. The upper layer was transferred into a vial for gas chromatography (GC) testing.
Gas chromatography (GC) analysis was conducted on a fused silica column SLB-5 ms (15 m × 0.25 mm × 0.10 μm). The column temperature increased from 50 oC (hold time 1 min) to 240 oC at 10 oC/min. The temperature of the flame-ionization detector was 250 oC. The flow rate of helium, the carrier gas, was 2.54 mL/min. A split injection mode was used with a split ratio of 1:30.
Crystallization
An X-ray diffractometer (XRD, PANalytical Empyrean with a PIXcel3D detector) was employed to identify crystalline substances present in unburned and burned specimens. A small amount of specimen was cut with a sharp knife and put on the sample table. The sample was moved into the diffractometer after it was pressed and labelled. The specimen was then tested in a 2θ range of 5 to 60o at a voltage of 45 kV and current of 40 mA. Cu LFF HR was used as the X-ray tube.
Functional groups
Infrared spectroscopy (IR, IFS 66V/S, Bruker) was used to determine the change in functional groups after combustion at high temperature. A small amount of powdered bottom ash and unburned specimen were put on the sample table in the spectrometer. The images were scanned and obtained at room temperature, 22 oC, over a spectral range of 400 to 4000 cm-1.
The data from XRD and IR were exported and changed to PRN files and DAT files, respectively. Then, they were analyzed using the program OriginPro8.
RESULTS AND DISCUSSION
Carbohydrate Analysis
Glucose, xylose, arabinose, galactose, and mannose were used as the standards to evaluate the location and contents of carbohydrates in unburned specimens and bottom ashes. Figure 1 presents GC images of unburned specimens and bottom ashes with 500 and 900 mL Si-Al compounds.
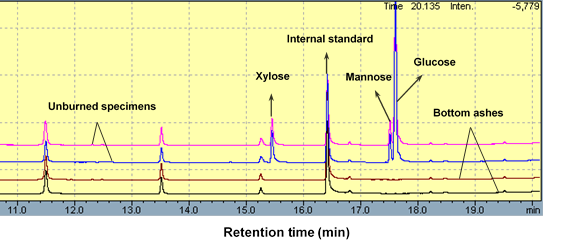
Fig. 1. Gas chromatogram (GC) images of both unburned specimens and burned specimens at 780 °C by cone calorimeter
For unburned specimens with 500 and 900 mL of Si-Al compounds, GC images showed almost the same intensity and retention time of peaks. Glucose, xylose, and mannose from holocellulose and likely corn starch (an ingredient of the glue used) were found at retention times ranging from 15 to 17 min. However, after combustion, monosaccharides disappeared in bottom ashes with 500 and 900 mL of Si-Al compounds. This suggested glucose, xylose, and mannose, which represent the elementary units of cellulose and hemicellulose, were indeed subjected to pyrolysis at high temperature, even with the protection of the Si-Al compounds.
Galactose and arabinose, which are another two types of sugar monomers from hemicellulose, are more sensitive and easier to be hydrolyzed under acidic conditions during manufacturing (Jin et al 2010). So they were not detected in the GC profiles of all the specimens. When retention time was about 15.25, one small peak appeared, and it could be arabinose. But the arabinose here probably resulted from the old GC analyzer, and it was not from fibers in the specimens. The contents of monosaccharides before and after combustion are listed in Table 1.
Carbohydrate content of unburned specimens ranged from 26.75% to 33.66%. Glucose and xylose were the two main monosaccharides in the fibers. The percentage of fiber in the specimens increased with decreasing dosage of Si-Al compounds when all other factors were held constant during manufacturing. Because of the higher percentage of fibers, the contents of glucose, xylose, and mannose in specimens with label “a” were higher than those with label “b”. The two types of raw materials showed a small amount of differences in carbohydrate contents. However, the effects of combustion on carbohydrate contents in two types of fibers were almost consistent. When the temperature was more than 200 oC, wood and cellulose isolated by several different fire retardants were subjected to thermal degradation (Levan and Winandy 1990). Pyrolysis is an exothermic reaction and tends to be self-sustaining once started. Especially for the materials made from fibers with ultra-low density, the pyrolysis rate is faster than that of solid wood. Even if fire retardants such as chlorinated paraffin and the Si-Al compounds used in this study significantly restrained fire intensity and volatiles release (Niu et al. 2014), the pyrolysis of carbohydrate was still obvious, as can be seen in Fig. 1 and Table 1. Combustion primarily generates carbon, water, carbon oxides, and hydrocarbons.
Table 1. Carbohydrate Content of Both Unburned Specimens and Burned Specimens at 780 °C by Cone Calorimeter
Crystallization
X-ray diffractometry (XRD) was applied to characterize the crystal structure, diffraction pattern, and particle size of ultra-low-density fiberboards during combustion. XRD profiles of four specimens from two types of raw materials with 500 and 900 mL of Si-Al compounds are given in Fig. 2.
Because unburned specimens contained both fibers and Si-Al compounds, their diffraction peaks in Fig. 2 were complicated and interesting. The peaks were from corporate contributions of fibers with crystalline cellulose at around 2θ = 22o (Lionetto et al. 2012) and Si-Al compounds with a crystalline phase at 2θ = 18.6o and a range from 25o to 60o, similar to crystalline zeolite (Kokotailo and Fyfe 1995; Kosanović et al. 2008). Furthermore, the intensity of diffraction peaks at 18.6o (2θ) and 25 to 60o (2θ) increased, and the intensity at 22o (2θ) decreased with a higher percentage of Si-Al compounds in the specimens, according to the comparisons between Fig. 2 (1-a) and (1-b), (2-a), and (2-b). After combustion at 780 oC by cone calorimeter, carbohydrate components were pyrolyzed and the crystalline structure of Si-Al compounds was destroyed or reorganized (Kokotailo and Fyfe 1995). Therefore, the diffraction peak from crystalline cellulose and the maximum peak at 18.6o (2θ) noticeably disappeared. Meanwhile, other sharp peaks became smaller and broader. The most significant broad diffraction pattern emerged at 2θ from 17o to 30o in the XRD profiles of fly ash and bottom ash, reflecting amorphous substances.
Fig. 2. Diffraction intensity of unburned and burned ultra-low-density fiberboards. 1- Kraft pulp; 2-Newspaper; a-500 mL Si-Al compounds; b-900 mL Si-Al compounds
The change from a narrow and sharp crystalline phase to a broad and blunt amorphous phase was also attributed to smaller crystalline size and lower amount of crystalline substances in the specimens (Gajović et al. 2008) caused by pyrolysis. Amorphous carbon, amorphous silicon oxide, and amorphous aluminum oxide might remain in the ash. These substances are very important for decreasing the amount of heat released during pyrolysis of carbohydrates. At the same time, some new crystalline substances were also detected from the XRD profiles of ash, especially in Fig. 2 (2-b), e.g., diffraction angles located at 12.4°, 14.5°, 25.5°, 28.3°, and 31.4°. These may have been the results of the interaction among amorphous substances or caused by re-arrangement of the crystalline structure at high temperatures. Furthermore, because the crystalline structure was subjected to less damage because of a lack of oxygen, the diffraction intensity of bottom ash was higher than that of fly ash.
Functional Groups
Functional group changes caused by combustion were analyzed through the IR spectra shown in Fig. 3. Several main peaks, at roughly 3342, 2916, 1726, 1022, and 478 (447) cm-1, can be seen for the unburned specimens. An absorbance peak between 3660 and 3060 cm-1, with a maximum near 3342 cm-1, is characteristic of a hydroxyl group typical of carbohydrates from fibers (Nishimiya et al. 1998). A band at 2916 cm-1 can be attributed to the CH stretching mode of methyl and methylene groups in carbohydrates. Ketone and aldehyde groups from carbohydrates were also detected at 1726 cm-1 or at 1705 cm-1 (Niu et al. 2011). A band from 1200 to 950 cm-1, with a maximum near 1026 cm-1, resulted from the stretching vibration of Si-O and Si-O-Si (Nayak and Singh 2007; Prado et al. 2010). A broad absorption from 1000 to 400 cm-1 was due to the stretching vibration of Si-O-Al, Al-O, and Si-O (Nayak and Singh 2007).
Compared to the specimens with label a, the specimens with label b had stronger absorbance at 1000 to 400 cm-1 because of a higher dose of Si-Al compounds. There were also differences in absorption intensity and absorption pattern for the two different raw materials. The specimens with kraft pulp as the matrix had stronger absorbance than those with newspaper in the range from 4000 to 400 cm-1, and their absorption pattern became broader at wavenumbers from 1200 to 400 cm-1. Nonetheless, kraft pulp and newspaper had similar effects on infrared absorbance.
Fig. 3. IR spectra of unburned and burned specimens. 1- Kraft pulp; 2-Newspaper; a-500 mL Si-Al compounds; b-900 mL Si-Al compounds
After the specimens were burned at 780 °C, their absorption peaks at 3342, 2916, and 1726 cm-1 disappeared. Three broad peaks were detected at 1591, 1022, and 478 (474) cm-1. One absorption band at 1591 cm-1 was caused by the vibration of aromatic C-C bonds from bio-char (Pӑrpӑriţӑ et al. 2014). Meanwhile, two other bands ranging from 1200 to 400 cm-1 were induced by the vibration of the Si-Al compounds. They became broader and higher than those of unburned specimens.
These results suggest that carbohydrate components were almost completely pyrolyzed at high temperatures. Also, the percentage of Si and Al compound in burned specimens increased, corresponding with the results for carbohydrate content and crystallization. Si-O and Al-O compounds formed after the crystalline structure was rearranged, and made a noticeable contribution to the suppression of heat and volatiles released during combustion of ULDFs.
CONCLUSIONS
- After combustion by cone calorimeter at 780 °C, glucose, xylose, and mannose detected from the GC profiles of ultra-low-density fiberboards (ULDFs) disappeared in bottom ashes. The diffraction peak representing Si-Al compounds at 18.6o (2θ) and crystalline cellulose at 22o (2θ) disappeared, and XRD profiles of fly ash and bottom ash became smaller and broader. Characteristic functional groups in fibers, such as hydroxyl, methyl, methylene, ketone, and aldehyde, disappeared, and absorbance of Si-O, Al-O, Si-O-Si, and Si-O-Al groups from Si-Al compounds located at 1200 to 400 cm-1 became stronger and broader.
- Therefore, carbohydrates in ULDFs were almost completely pyrolyzed, even under the conditions of incomplete combustion. The crystalline structure of Si-Al compounds was rearranged and probably generated more amorphous substances (such as silicon oxide and aluminum oxide) during combustion, thus resulting in higher absorption peaks in IR profiles.
- The release amounts of heat and volatiles decreased, attributing to generation of more amorphous Si-O and Al-O compounds.
ACKNOWLEDGMENTS
The authors are grateful for the support of ÅForsk in Stockholm, Grant. No. 24924.
REFERENCES CITED
Cervin, N. T., Andersson, L., Sing Ng, J. B., Olin, P., Bergström, L., and Wågberg, L. (2013). “Lightweight and strong cellulose materials made from aqueous foams stabilized by nanofibrillated cellulose,” Biomacromolecules 14(2), 503-511. DOI: 10.1021/bm301755u
Chen, T. J., Niu, M., Xie, Y. Q., Wu, Z. Z., Liu, X. Z., Cai, L. L., and Zhuang, B. R. (2015). “Modification of ultra-low density fiberboards by an inorganic film formed by Si-Al deposition and their mechanical properties,” BioResources 10(1), 538-547. DOI: 10.15376/biores.10.1.538-547
Frenette, D., Roy, B., Cadieux, S., Labbe, M., and St-Cyr, S. (1996). “Cellulosic fiber insulation material,” United States Patent No. 5516580.
Gajović, A., Gracin, D., Djerdj, I., Tomašić, N., Juraić, K., and Su, D. S. (2008). “Nanostructure of thin silicon films by combining HRTEM, XRD and Raman spectroscopy measurements and the implication to the optical properties,” Applied Surface Science 254(9), 2748-2754. DOI: 10.1016/j.apsusc.2007.10.014
ISO 5660, International Organization for Standardization (2002). “Fire tests-Reaction to fire-Heat release, smoke production and mass loss rate-Part 1: Heat release rate (Cone Calorimeter method).”
Jin, Q., Zhang, H. M., Yan, L. S., and Huang, H. (2010). “Dilute acid hydrolysis reaction of biomass hemicellulose,” Progress in Chemistry 22(4), 654-662.
Karlsson, O., Torniainen, P., Dagbro, O., Granlund, K., and Morén, T. (2012). “Presence of water-soluble compounds in thermally modified wood: Carbohydrates and furfurals,” BioResources 7(2), 3679-3689. DOI: 10.15376/biores.7.2.3679-3689
Kawasaki, T., Zhang, M., and Kawai, S. (1998). “Manufacture and properties of ultra-low-density fiberboard,” Journal of Wood Science 44(5), 354-360. DOI: 10.1007/BF01130447
Kokotailo, G. T., and Fyfe, C. A. (1995). “Zeolite structure analysis with powder X-ray diffraction and solid-state NMR techniques,” The Rigaku Journal 12(1), 3-10.
Kosanović, C., Bosnar, S., Subotić, B., Svetličić, V., Mišić, T., Dražić, G., and Havancsák, K. (2008). “Study of the microstructure of amorphous aluminosilicate gel before and after its hydrothermal treatment,” Microporous and Mesoporous Materials 110(2-3), 177-185. DOI: 10.1016/j.micromeso.2007.06.007
Levan, L. S., and Winandy, J. E. (1990). “Effect of fire retardant treatments on wood strength: A review,” Wood and Fiber Science 22(1), 113-131. DOI: 10.3923/ijar.2008.331.339
Lionetto, F., Sole, D. R., Cannoletta, D., Vasapollo, G., and Maffezzoli, A. (2012). “Monitoring wood degradation during weathering by cellulose crystallinity,” Materials 5(10), 1910-1922. DOI: 10.3390/ma5101910
Liu, J. H. (2013). Fire Retardant Properties and Mechanism of Ultra-Low Density Wood Fiber-Based Material, Ph.D. dissertation, Fujian Agriculture and Forestry University, Beijing, China.
Nayak, S. P., and Singh, B. K. (2007). “Instrumental characterization of clay by XRF, XRD and FTIR,” Bulletin of Materials Science 30(3), 235-238. DOI: 10.1007/s12034-007-0042-5
Nishimiya, K., Hata, T., Imamura, Y., and Ishihara, S. (1998). “Analysis of chemical structure of wood charcoal by X-ray photo electron spectroscopy,” Journal of Wood Science 44(1), 56-61. DOI: 10.1007/BF00521875
Niu, M., Zhao, G. J., and Alma, M. H. (2011). “Thermogravimetric studies on condensed wood residues in polyhydric alcohols liquefaction,” BioResources 6(1), 615-630.
Niu, M., Hagman, O., Wang, X. D., Xie, Y. Q., Karlsson, O., and Cai, L. L. (2014). “Effect of Si-Al compounds on fire properties of ultra-low density fiberboard,” BioResources 9(2), 2415-2430. DOI: 10.15376/biores.9.2.2415-2430
Pӑrpӑriţӑ, E., Brebu, M., Uddin, M., Yanik, J., and Vasile, C. (2014). “Pyrolysis behaviors of various biomasses,” Polymer Degradation and Stability 100(2), 1-9. DOI: 10.1016/j.polymdegradstab.2014.01.005
Prado, L., Sriyal, M., Ghislandi, M., Barros-Timmons, A., and Schulte, K. (2010). “Surface modification of alumina nanoparticles with silane coupling agents,” Journal of the Brazilian Chemical Society 21(12), 2238-2245. DOI: 10.1590/S0103-50532010001200010
Theander, O., and Westerlund, E. (1986). “Studies on dietary fiber. 3. Improved procedures for analysis of dietary fiber,” Journal of Agricultural and Food Chemistry 34(2), 330-336. DOI: 10.1021/jf00068a045
Wang, X. C., Shi, S. L., Cheng, Z. Q., Hou, H. M., Mo, X. H., and Tian, J. (2000). “Synthesis of (Si-, Al-) ceramic wood by chemical process,” Chinese Journal of Materials Research14(1), 51-55. DOI: 10.1007/s11632-008-0039-1
Xie, Y. Q., Chen, Y., and Zhang, B. G. (2004). “Study on a formed material from plant fibers,” China Wood Industry 18(2), 30-32. DOI: 10.3969/j.issn.1001-8654.2004.02.009
Xie, Y. Q., Chen, Y., and Wei, Q. H. (2008a). “Study on forming a truss-like reticular structure made from nature fiber under the effect of liquid frothing,” Journal of Fujian College of Forestry 28(3), 299-303. DOI: 10.3969/j.issn.1001-389X.2008.03.003
Xie, Y. Q., Tong, Q. J., and Chen, Y. (2008b). “Construction mechanism of reticular structure of plant fiber,” Journal of Korea Furniture Society 19(2), 106-110. DOI:
Xie, Y. Q., Tong, Q. J., Chen, Y., Liu, J. H., and Lin, M. (2011). “Manufacture and properties of ultra-low density fiberboard from wood fiber,” BioResources 6(4), 4055-4066. DOI: 10.15376/biores.6.4.4055-4066
Xu, J. Y., Sugawara, R., Widyorini, R., Han, G. P., and Kawai, S. (2004). “Manufacture and properties of low-density binderless particleboard from kenaf core,” Journal of Wood Science50(1), 62-67. DOI: 10.1007/s10086-003-0522-1
Zicherman, J. B., and Williamson, R. B. (1981). “Microstructure of wood char. Part I: Whole wood,” Wood Science and Technology 15(4), 237-249. DOI: 10.1007/BF00350942
Zhou, P., Zhang, Z. Y., and Liang, S. P. (2000). “Combination of Populus tomentosa wood and inorganic substances,” Journal of Beijing Forestry University 22(6), 39-42. DOI: 10.3321/j.issn:1000-1522.2000.06.010
Article submitted: January 6, 2015; Peer review completed: March 22, 2015; Revised version received and accepted: March 23, 2015; Published: March 27, 2015.
DOI: 10.15376/biores.10.2.2903-2913
