Abstract
“Green” resole phenolic resins for laminating applications were synthesized, and their properties and thermal stability were determined. The plant-based cardanol and condensed tannin were used as the partial substitutes of up to 40% of the phenol in the synthesis of the phenolic resins. The resins were synthesized with different proportions of phenol (P) to cardanol (C) and with different total molar ratio to the formaldehyde (F) in the resins (1.25 to 2.0). An increased cardanol content resulted in a proportional increase in the flexibility and fracture toughness of the cured cardanol-phenol-formaldehyde (CPF) resins. Also, a direct proportionality was found between increasing cardanol content and decreased crosslink density of the CPF resins. The best results were obtained with the formulation with a P:F molar ratio equal to 1:1.25. Tannin was incorporated into the CPF resins and the fracture toughness and flexibility values of the cured Tannin-CPF resins were found to be proportional to and increasing with the tannin content. However, glass transition temperature (Tg), flexural stress, and flexural modulus values of the CPF resins decreased with the tannin content. TGA-FTIR study of the resins was carried out and the emitted gas species during the pyrolysis of the samples were identified. The thermal stability and the temperature of degradation of the cured CPF resins decreased with increasing cardanol content.
Download PDF
Full Article
Characterization of Environmentally Sustainable Resole Phenolic Resins Synthesized with Plant-based Bioresources
Francisco Cardona* and M. T. H. Sultan
“Green” resole phenolic resins for laminating applications were synthesized, and their properties and thermal stability were determined. The plant-based cardanol and condensed tannin were used as the partial substitutes of up to 40% of the phenol in the synthesis of the phenolic resins. The resins were synthesized with different proportions of phenol (P) to cardanol (C) and with different total molar ratio to the formaldehyde (F) in the resins (1.25 to 2.0). An increased cardanol content resulted in a proportional increase in the flexibility and fracture toughness of the cured cardanol-phenol-formaldehyde (CPF) resins. Also, a direct proportionality was found between increasing cardanol content and decreased crosslink density of the CPF resins. The best results were obtained with the formulation with a P:F molar ratio equal to 1:1.25. Tannin was incorporated into the CPF resins and the fracture toughness and flexibility values of the cured Tannin-CPF resins were found to be proportional to and increasing with the tannin content. However, glass transition temperature (Tg), flexural stress, and flexural modulus values of the CPF resins decreased with the tannin content. TGA-FTIR study of the resins was carried out and the emitted gas species during the pyrolysis of the samples were identified. The thermal stability and the temperature of degradation of the cured CPF resins decreased with increasing cardanol content.
Keywords: Phenolic PF; Flexural; Fracture toughness; Cardanol; Tannin; SEM; DMA; TGA-FTIR
* Corresponding author: francisco.c@upm.edu.my; thariq@upm.edu.my
INTRODUCTION
The chemistry of renewable resources and the production of plant-based environmentally-friendly polymers have increasingly become the focus of industrial and academic researchers around the world. This is due to the depleting stocks of petrochemical products and the growing pollution and environmental concerns associated with synthetic materials. Phenolic resins have been known as commercial synthetic polymer materials since the discovery of the moldable formulation from the reaction of phenol and aldehydes in 1907, by Backeland (1909). These synthetic resins have been used extensively in the production of thermosets, adhesives for wood products, pre-pegs, resin transfer molding, honeycomb structures, and aerospace components (King et al. 1974; Knop and Pilato 1985; Mottram et al. 1992; Shafizadeh et al. 1999).
Typically, phenolic resins are prepared by the reaction of formaldehyde (F) with phenol (P). Depending on the F:P molar ratio and the type of catalyst, the reaction leads to either a liquid resole or a solid powder novolak type of phenolic resin. Phenolic resins are thermosetting in nature and have properties of high-temperature resistance, infusibility, and low smoke emissions under fire. Structures are widely variable depending on synthesis conditions such as the mole ratio of reactants, the reaction time and temperature, water content, and residual formaldehyde content.
The research work presented here is focused on the modification of liquid resole phenolic resins with natural renewable resources, which were prepared under alkaline conditions and with an excess of formaldehyde, F:P molar ratio > 1.0, as previously reported (Yeddanapalli et al. 1968; Knop and Pilato 1985). Phenolic resin properties can also be modified by reacting phenol with other aldehydes, using substituted phenols, etherification of phenol, and incorporation of organic liquids. Phenol-formaldehyde (PF) resins have high thermal and chemical resistance, but they are brittle and have relatively low tensile strength (So and Rudin 1990; Wang et al. 1997; Seena et al. 2002). This property limits their applications in fiber-reinforced laminates and in composites for aerospace and civil engineering structures.
This research investigation focused on modifying resole phenolic resins with plant-based substitutes of phenol, in particular, cardanol, obtained from cashew nut shells, and condensed tannin, extracted from the bark of trees. The purpose of these modifications was to increases the environmental-friendly profile of the phenolic resins and to enhance their properties and performance in comparison with their synthetic Phenol-Formaldehyde (PF) counterparts. Resins incorporating cardanol have a similar chemical profile to the PF resins (see Figs. 1 and 2), and have a comparatively higher flexibility and lower cost (Attanasi et al. 1996). Therefore, liquid phenolic resins prepared by the combination of cardanol and phenol-formaldehyde (CPF) should overcome most of the physical disadvantages of neat PF resins. This study further increases the “green” and biodegradable profile of the resole phenolic resins by incorporating diluted condensed wattle tannin.
Cardanol is an industrial-grade yellow oil obtained by the distillation of cashew nut shell liquid (CNSL), a phenolic-based by-product of the cashew nut (Anacardium occidentale) industry. In its natural form, crude CNSL is a mixture of different phenolic compounds, with an alkyl-phenolic oil contained in the spongy mesocarp of the nut. CNSL derived from the most diffused roasted mechanical processes of the cashew industry represents nearly 25% of the total nut weight, and its production worldwide (Africa, Asia, and South America being the main producers) is estimated to be about 300,000 tons per year (Attanasi et al. 1995). The main constituents of CNSL are cardanol, cardol, 2-Methyl cardol, and small amounts of anacardic acid (Fig. 1).
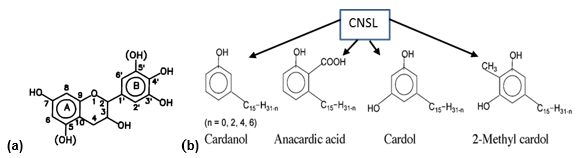
Fig. 1. Chemical structure of (a) the natural wattle tannin, and (b) components of CNSL
The side chain characterizing this phenolic compound can be saturated, mono-, bi– or tri-olefinic with the double links, respectively, in positions (8), (8, 11), or (8, 11, 14), and with an average value of one double bond per molecule (Attanasi et al. 1996).
CNSL is a sustainable, low cost, and widely available natural resource by-product of the cashew industry. Therefore, CNSL, as well as cardanol and its derivatives, are important starting materials having various industrial utilizations, such as additives for lubricants and diesel engine fuels, asbestos-free brake pads in automobiles, antioxidants, stabilizers, flame retardants, resins, inks, hydro-repellents, and chemical intermediates (Attanasi et al. 1995; Filippone et al. 2002; Mele et al. 2004; Balachandran et al. 2013; Bloise et al. 2014).
In this study, for the first time, phenolic resole resins were synthesized by partially substituting phenol with added cardanol (CPF resins), and additionally by incorporating tannin-cardanol into the phenolic resins (tannin-CPF resins). The proposed synthesis mechanism of the CPF resins, including the intermediate species and the final cross-linked structure, is presented in Fig. 2.
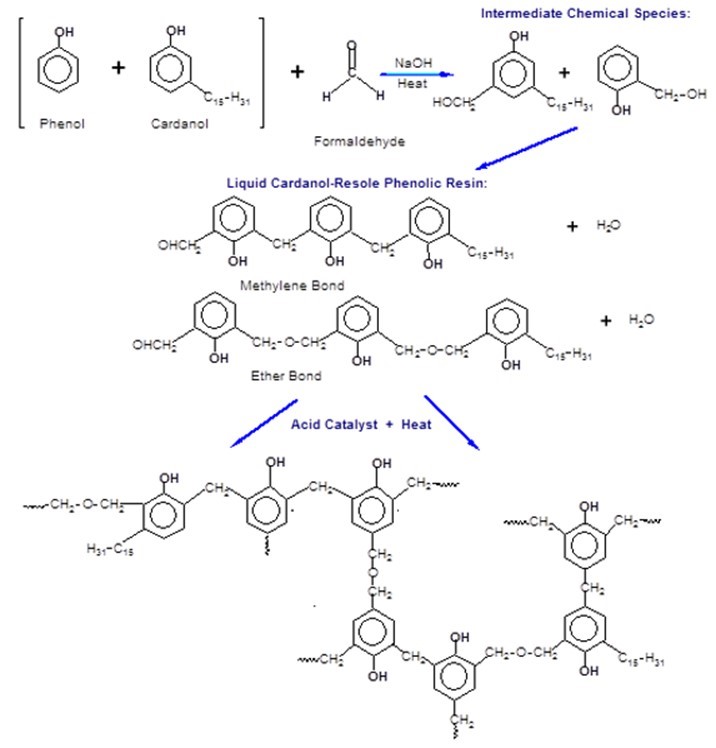
Fig. 2. Mechanism of the chemical synthesis and the curing of the CPF resins
Table 1. Chemical Composition of Wood and Bark from Cedar Tree Species
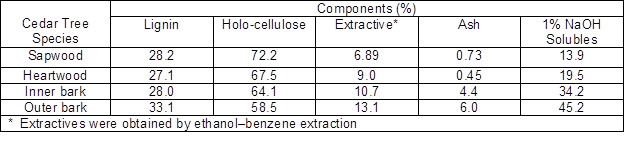
Bark is a valuable natural resource obtained worldwide as a by-product of the timber industry. Bark differs from wood in terms of chemical compositions. Generally, bark consists of polysaccharides (cellulose, hemicelluloses), pectic substances, phenolic polymers (including lignin and high molecular weight tannins), and cross-linked polyesters (such as suberin and cutin). The holocellulose in bark generally contains a higher proportion of mannose, and the lignin in some conifer barks can be more highly cross-linked than wood lignin. In addition, some low molecular weight components, such as low molecular weight phenolics, fatty acids, and resins, can also be found in the bark (Hon and Shiraishi 2001). The chemical composition of bark varies by tree species (hardwood or softwood), tree parts (root, stem, or branch), tree stress (normal wood, tension wood, or compression wood), the geographic location, climate, and soil conditions.
As shown in Table 1 for the cedar tree species, the lignin content of the bark is usually higher than that of the wood part. Also, compared with wood, bark contains more extractives and ash. Most of the extractives in bark are similar to those contained in the wood; the main difference is that many barks contain more polyphenols and suberin of a high molecular weight (Usia and Kara 1997; Pappa et al. 2000; Chow et al. 2008). Additionally, bark is not a favorable feedstock for the production of fermentable sugars because it contains a high content of lipophilic extractives, as well as aromatic compounds including tannin and lignin (Torget et al. 1991; Kim et al. 2005).
In contrast, the aromatic polyol structure components in bark (condensed tannin) favor its utilization in PF resins and foam production (Feng et al. 2013). Precisely the polyphenol chemical structure of condensed tannin (Fig. 1) makes this natural resource fully compatible and miscible with phenolic resins. Tannins are commonly extracted from the bark with a solution of 1 to 2% NaOH in water at 80 to 90 oC and with continuous stirring for 6 to 8 h (Vázquez et al. 1996). Temperature and alkali type greatly affect extractive yield: extraction efficiency using Na2CO3 is much lower than extraction with NaOH (Liiri et al. 1982). The highest condensable polyphenol content (27.3% of bark) of spray-dried extractives was obtained by extraction at 90 oC in 5% NaOH solution with a solid to liquid ratio of 1/6 (Pohjamo et al. 2003). The extracted tannins are naturally occurring phenolic compounds, which have been extensively investigated finding numerous industrial applications (Pizzi et al. 2009). Tannins represent the best substitute for phenol in phenolic resins preparation, with the advantage of being cheaper and also having a higher reactivity towards formaldehyde than phenol. For those reasons tannin is the renewable resource that is most widely used in the production of phenolic adhesives. Traditionally, tannin is used to transform animal skins and hides into leather. This process is achieved through the reaction between the hydroxyl groups of tannins and the peptide bonds in amino acids present in animal protein. In addition, tannin has applications in tiles and floor manufacturing, as well as in drilling muds used to drill oil wells (Feng et al. 2013). Studies on tannin-based adhesives date back to 1950s; wattle bark tannin-based adhesive has been commercially used for exterior-grade particleboard for the last forty years (Pizzi 1982). Tannins from the barks of spruce (Pizzi and Merlin 1981), pine (Pizzi 1982), Nigerian woods (Vázquez et al. 2001), Taiwan acacia, and China fir (Lee and Lan 2006) have also been extensively applied in preparing tannin adhesives. Most of the previous research on the utilization of tannin in resole phenolic resins has been aimed at their applications in adhesives formulations for the timber industry. However, the simultaneous use of tannin and cardanol has not been previously reported for either phenolic formulations aimed to laminating applications or for the development of “green” biodegradable phenolic resins. Therefore, the contribution of this work to the field of composite materials is that present for the first time the characterization and effects on the properties of resole phenolic resins of the simultaneous incorporation of increasing amounts of tannin and cardanol into the resins formulation for molding and laminating applications.
EXPERIMENTAL
Synthesis of CPF and Tannin-CPF Resins
The liquid resole CPF resins were prepared with different mixtures of cardanol (received from Satya Cashew Chemicals Ltd., Tamil Nadu, India) and phenol reacting with formaldehyde in the presence of an alkaline catalyst. Phenol was replaced with cardanol up to 40% by weight in the synthesis. The reactions were carried out in a glass reactor equipped with a stirrer, a condenser, and an internal heating unit. The required amounts of phenol (88% solution of phenol in water from Huntsman Chemical Company, Brimbank, Victoria, Australia), cardanol, and paraformaldehyde were mixed by keeping the molar ratio of total phenol (phenol plus cardanol) to formaldehyde at 1:1.25 for the first set of resins (CPF resin I), 1:1.5 for the second set (CPF resin II), and 1:2.0 for the third set (CPF resin III). A catalyst, consisting of an aqueous solution of NaOH-45% by weight was added to the reaction mix (4%, w/w on the basis of total phenol), which is an optimal amount of the catalyst for the reaction, as previously reported (Cardona and Moscou 2009). With continuous stirring of the mixture, the temperature was maintained 60 oC for 1 h, then rose to 80 oC for 1 h, and finally fell back to 60 oC for 1 h. The chosen acidic catalyst for curing the CPF and the CPF/PF resin blends was a mixture of xylene-sulfonic and phosphoric acids with water (Phencat10 catalyst from Hexion Ltd., USA), which cures the phenolic resins relatively slowly and therefore enables better mechanical properties to be achieved. An amount of 3 wt% of the catalyst was used for the curing of the liquid phenolic resins.
The condensed tannin (Bondtite-345 from Bondtite Ltd., Botany, NSW, Australia) consisted of a brown powder with a moisture content of approximately 10% by weight. Prior to mixing with the liquid phenolic CPF resins, the tannin was dissolved in glycerol in the presence of sulphuric acid (1% by weight). Initially the glycerol and the sulphuric acid were mixed at 80 °C, followed by the slow addition of tannin while continually stirring the solution. The mixture maintained 80 oC for 40 min. The tannin was dissolved in the acid-glycerol in concentrations of 10%, 20%, 30%, and 40% by weight (mixes B, C, D, and E, with mix A as the acid-glycerol without tannin). The dissolved tannin was a sticky, brown liquid with its viscosity increasing with the tannin content. To obtain the tannin-CPF resole resins, the dissolved tannin was added in different amounts (% weight) to the CPF resins, then continuously stirred for 10 min. The liquid resins were cured with an acidic catalyst consisting of phosphoric acid (20%), xylene-sulfonic acid (70%), and water (10%) (Phencat15 catalyst from Hexion Ltd.). After adding the catalyst to the resins and mixing for 5 min, the resins were poured inside of square molds 15×15 cm, 12 mm thick. The test specimens were initially cured at room temperature for 12 h, followed by post-curing inside of a laboratory oven at 80 °C for 4 h. Simultaneous Thermogravimetric-Fourier Transform Infrared Spectroscopy (TGA-FTIR) analysis, scanning electron microscopy (SEM) analysis, and dynamic mechanical analysis (DMA), as well as mechanical, and fracture toughness tests of the post-cured tannin-CPF resins were performed.
Viscosity Measurements
Viscosity values (cP) were established with a Brookfield LV viscometer (Model 94800-0 from LT Scientific, USA) and using the instrument spindle # 10. Tests were conducted at room temperature (24 oC) and with a shear rate of 1s-1. The solid content (%) of the tannin-CPF resins was measured by the difference in weight of the samples taken before and after exposure to 150 °C for 2 h inside of the laboratory oven.
Mechanical Tests
After post-curing, the CPF and tannin-CPF resin samples were tested for flexural stress (MPa), elongation at break (%), and flexural modulus (MPa), taking the average value of five tests for each sample in accordance with the ISO standard 178 (2010), which specifies a method for determining the flexural properties of rigid and semi-rigid plastics under defined conditions. Mechanical tests were performed using a MTS Alliance RT/10 machine (10 kN machine capacity) from MTS Systems Corporation, USA. Fracture toughness tests were performed using the same MTS 10 kN machine by conventional three-point bending test and in accordance with ISO standard 17281 (2002), which is entitled Plastics – Test Method for Determination of Fracture Toughness. Each one of the five CPF resin samples were notched in such a way that a/w » 0.5 (see next). The critical stress intensity factor, KIc, is representative of the fracture toughness and it was calculated according to Eq. 1 (Dharmarajan and Vipulanandan 1988),
KIc = f(a/w) (1)
where F is the load at break (N), h is the thickness of the specimen, w is the width of the specimen, a is the notch length, and f is a geometry factor for the specimen and the notch. When the specimen is assumed to have infinite width, then f = 1. Since the widths of the specimens were not infinite, then f = 1.1. All three-point bending tests were performed in an electro-mechanic testing machine equipped with a 10 kN capacity load cell, using a support span of 64 mm with a cross-head speed of 2.0 mm/min.
Simultaneous TGA/FTIR Analysis
The analyses were performed using an integrated TGA-IR Module for Nicolet FT-IR Spectrometers with an incorporated Evolved Gas Analysis system. This system consists of a TA Instruments Q500 Model thermogravimetric analyzer system (TGA) fitted with an Interface Unit connected to the gas-cell of a Thermo Nicolet NEXUS 670 FTIR spectrometer, from GMI Ltd, Minnesota, USA. Infrared (IR) spectral signals and thermogravimetric data were acquired every 10 sec with a two-computer workstation as the sample was heated in the furnace of the TGA at 10 °C/min under a positive flow of nitrogen gas (100 mL/min). For the TGA-FTIR analysis, solid samples of the cured CPF resins (ca. 40 mg) were used. The gas-IR cell and the transfer-line between the TGA and the FTIR instruments were held at 220 °C to avoid condensation of gases. Spectral searches were done using the OMNICTM Mercury TGA real-time Search-Master software (from Thermo Scientific Inc, USA) incorporated into the FTIR Nicolet Spectrometer system and with its vapor phase library and algorithms.
Scanning Electron Microscopy Analysis
A JSM-6500F (from JEOL Ltd. Japan)) field emission scanning electron microscope (SEM) operating at 10 kV was used to examine the cured solid resins morphologies. The specimens were washed several times with tetrahydrofuran and distilled water and finally dried in air. All samples were thinly sputter-coated with Pt–Au using a Polaron E5100 Sputter Coater (from Quorum Technologies Ltd., UK). The morphologies exhibited regular porous domains imbedded in a solid matrix phase.
RESULTS AND DISCUSSION
Fracture Toughness, Solid Content, and Crosslink Density
The critical stress intensity factor KIc (MPa m1/2) indicates the toughness of the cured resins (Yosibash et al. 2004). For all levels of cardanol content up to 40%, the fracture toughness of the samples prepared with the CPF resin I was higher than that of the neat PF phenolic resin (0.9 MPa m1/2) (see Fig. 3a).
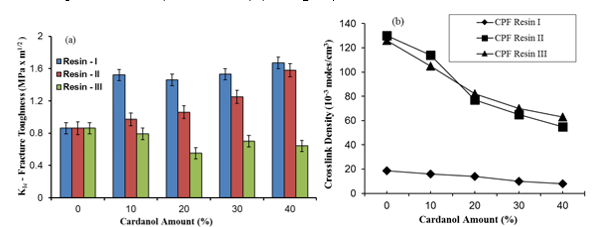
Fig. 3. (a) Fracture toughness factor (KIc) of the cardanol-modified CPF resins (I, II, and III), and (b) crosslink density of the synthesized CPF resins vs. cardanol content
In particular, at 40% cardanol content, the fracture toughness factor (KIc) value was similar for the CPF resins I and II. This indicated that at 40% cardanol content, the difference of the P:F molar ratio between resin I (1.25) and resin II (1.50) did not significantly affect the value of fracture toughness of the synthesized CPF resins. However, the fracture toughness factors of the samples with resin III (P:F ratio of 1:2) were lower than the value of the neat PF phenolic resin, due to the higher brittleness of the resin III samples compared with the other two CPF resins. The high brittleness of resin III with 40% of cardanol is due to the comparatively high crosslink density of this resin, as shown in Fig. 3(b). Figure 3(a) also confirmed the lower crosslink density value of resin I at all levels of cardanol content in comparison with resins II and III, which explains the high fracture toughness of resin I. Therefore, cardanol can serve not only as an effective substitute of phenol in the synthesis of phenolic resins but also as a toughening agent of the fully cured resins, providing a valid environmentally-friendly alternative in the synthesis of the resole phenolic resins.
The viscosity and solid content values as a function of the tannin content (20% tannin dissolved mix) in the synthesized CPF resin are shown in Fig. 4. Viscosity and solid content values increased linearly when dissolved tannin was added to the CPF resin. This behavior was a result of the large polyphenol chemical structure of tannin that yielded increasing corresponding values of viscosity and solid content in the resin network.
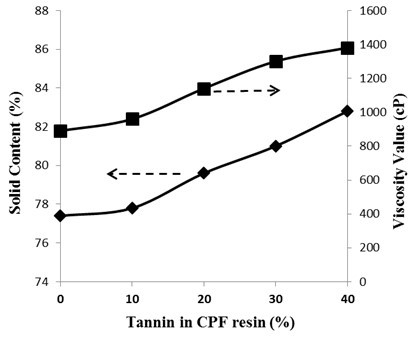
Fig. 4. Plots of the viscosity values (cP) and of the solid content (%) vs. tannin content (20 wt% of tannin dissolved) in the CPF-I resins
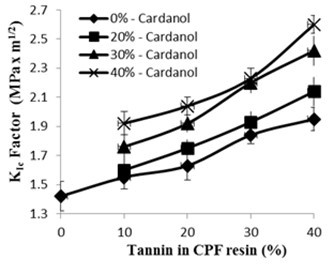
Fig. 5. Fracture toughness KIc factor (MPa.m1/2) vs. tannin content (20% dissolved tannin) of the CPF resins with different cardanol content
The fracture toughness, or critical stress intensity factor KIc (MPa.m1/2), for the tannin-CPF resins with different cardanol content is presented in Fig. 5. As shown in Fig. 5, the KIc factor increased with the amount of tannin added to the CPF resins. This enhanced fracture toughness of the CPF resins that increased with the amount of tannin may be attributed to a greater level of flexibility and ability for spatial rearrangement inside of the CPF phenolic thermoset network. Also, the addition of dissolved tannin to the phenolic network induced the relatively low crosslink density of the cured CPF resins, as previously mentioned.
Tannin has been demonstrated to have higher reactivity towards formaldehyde than phenol (Sumin and Hyun-Joong 2003), giving place to larger consumption of formaldehyde during the synthesis of tannin modified phenolic adhesives than in the reaction of solely phenol and formaldehyde. Therefore, the increase in the solid content of the CPF resins with the increase in tannin content should result in less free residual formaldehyde and less residual phenol in the phenolic resins after the synthesis (see Fig. 4). Moreover, the increase in the solid content value with the added amount of tannin indicates that the tannin-modified resole phenolic resins should be safer for structural engineering applications than the standard PF resins, due to the lower amount of volatile water and residual compounds of the former over the latter. The tannin-CPF resins would also produce less harmful emissions should laminates made with them be involved in a fire.
Crosslink Density and Tg Values of Tannin-CPF Resins
The crosslink density can be defined as the fraction of monomer units that are crosslinked (Galia et al. 1994). The crosslink density was calculated from the rubber elasticity theory, Eq. 2 (Gronski et al. 1992),
v= E/3RT (2)
where n represents the crosslink density (number of chemical crosslinks per cm3), R is the gas constant (8.314 J/K.mole), T is the temperature (oK), and E is the elastic modulus at Tg temperature value + 50 degrees taken from the DMA test of the materials (DMA Q800 Model from TA Instruments Ltd., Australia).
The crosslink density values of the tannin-CPF resins decreased with increasing tannin content, as shown in Fig. 6a. Moreover, the crosslink values of the tannin-modified CPF resins were significantly lower than that of the CPF and of the neat PF resins (140 x 10-3 moles/cm-3).
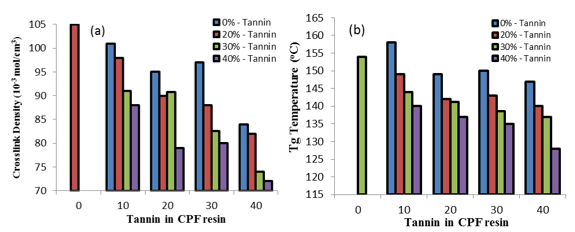
Fig. 6. (a) Crosslink density values vs. tannin content in the tannin-CPF resins, and (b) the glass transition temperature (Tg) values vs. tannin content in the CPF resins. Tg values were obtained from the top of the tand peak of the DMA tests.
Clearly, the presence of cardanol and tannin decreased the crosslink density of the cured phenolic resin network and thereby made the solid cured resins more flexible. This confirmed that the characteristic brittleness of PF resins was due to their high crosslink density (about 100 times higher than that of epoxy resins) and that the partial substitution of phenol with cardanol during the synthesis of the phenolic resin, plus the additional effect of tannin in the resins, produced a phenolic resin with lower brittleness and increased toughness.
A decrease in the values of crosslink density with the tannin content in the CPF resins also resulted in a decrease in the Tg of the tannin-CPF resins, as shown in Fig. 6b. Tg values were taken from the top of the tand peak of the DMA plots (Fig. 7). The observed effect of decreased Tg value with tannin content was larger for the CPF resins with the added mix E (40 % tannin content), as shown in Fig. 6b. This indicated a proportional relationship between the Tg value and the tannin content in the CPF resins.
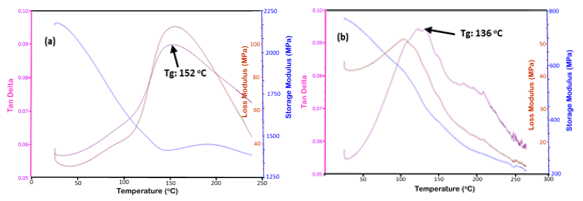
Fig. 7. Typical DMA plots of the cured tannin-CPF resins with (a) mix C (20% tannin), and (b) mix E (40% tannin)
Mechanical Properties
Figure 8 shows the results of the mechanical flexural tests of the CPF resins with tannin content in the resins.
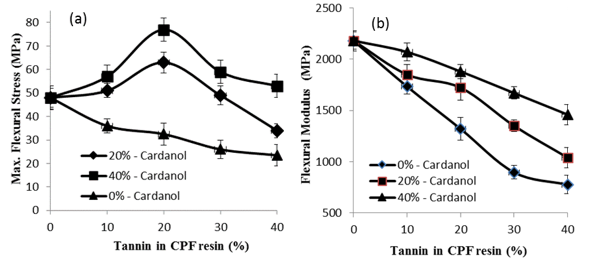
Fig. 8. Results of the mechanical flexural tests vs. tannin content (%) for the tannin-CPF resins. Before testing, the samples were cured at room temperature for 12 h, followed by post-curing at 100 oC for 4 h.
As shown, the value of maximum flexural stress of the CPF resins initially increased with the amount of tannin added up to 20%. Above 20% tannin addition, the flexural stress decreased, which is due to the extensive decrease in the crosslink density of the cured resins above 20% tannin content, as shown in Fig. 6(a).
The results indicated that small amounts of dissolved tannin had a beneficial effect on the flexural strength of the tannin-CPF resins, but a higher added quantity of tannin decreased the flexural performance of the CPF resins. The initial increase in the flexural stress was associated with the above mentioned decreases in the crosslink density of the cured tannin-containing CPF resins (Fig. 6a), which resulted in a less brittle resin. Figure 8 also shows that the amount of dissolved tannin had a large effect on the flexural modulus of the cured tannin-CPF resins. The flexural modulus decreased with increasing tannin concentrations in the CPF resins. This decrease was comparatively lower for the tannin-CPF resins with the higher quantity of dissolved tannin in glycerol (40%), which indicated that the polyphenol type of tannin compound acted as a toughening agent inside of the network of the cured CPF resins (Fig. 8).
Table 2 presents the results of the flexural tests of the blended CPF/PF resins with different weight ratios of the two phenolic resins (0 to 100%). The results are from the synthesized CPF resin I (P:F molar ratio 1:1.25) with cardanol content from 10% to 40% by weight. The CPF resin I showed the largest change in its ultimate mechanical properties compared with CPF resins II and III. This was due to its lower P:F molar ratio that resulted in a more stable and tightly cross-linked network upon curing.
Table 2. Results of the Flexural Tests of the Synthesized CPF Resin I with Different Cardanol Content (from 10% to 40%) and Blended with Phenol-Formaldehyde (PF) Resin (CPF/PF Blends)
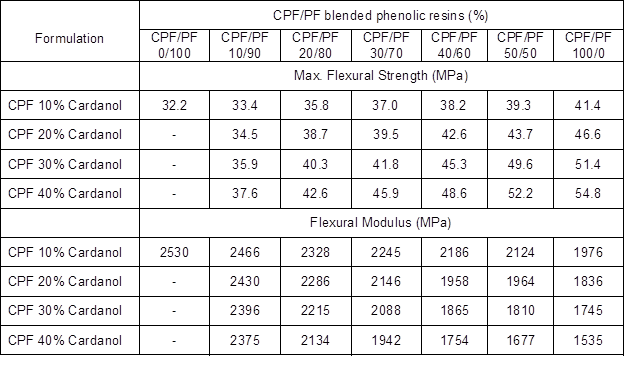
The values presented in Table 2 clearly indicate that the flexural modulus for the blended CPF/PF resins decreased with the CPF resin I content, whereas the max stress and the strain values increased with the amount of CPF resin I. The results confirmed a decrease in the level of brittleness of the phenolic-cured network, and that the changes in the mechanical properties of the CPF/PF blends were enhanced with the amount of cardanol present in CPF resin I. Therefore, the most flexible and tougher cured CPF/PF resin blend observed was the CPF resin I with 40% cardanol content. Further confirmation of the high flexibility of this CPF resin formulation was given by the fact that it showed low crosslink density value, as previously mentioned (Fig. 6a). In general, the tannin-CPF phenolic resole resins are more suitable than both the CPF and the neat synthetic commercial PF resins for civil engineering structures, such as manufacturing reinforced laminates and sandwich panels, due to their improved toughness and overall better mechanical performance.
Simultaneous TGA/FTIR Analysis
The 3D diagram of the IR spectra of the evolved gases (generated ‘waterfall’ with the spectral processing capabilities of the OMNIC Atlus software) of the CPF resin with 40% cardanol, emitted from the furnace of the TGA, is illustrated in Fig. 9. The strongest IR spectra were observed in the 40 to 50 min region, which correspond to the 400 to 500 oC temperature range in the TGA thermographs. This was also the point of the largest thermal induced decomposition and emission of the phenolic resin samples, as shown in the TGA graphs in Fig. 12.
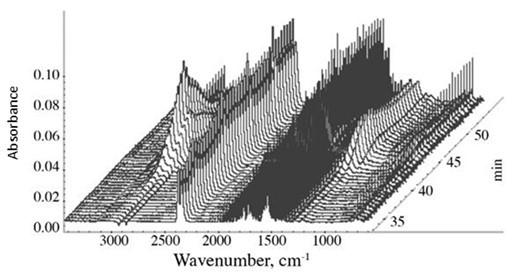
Fig. 9. 3D Infrared Waterfall of the CPF with 40% cardanol. The TGA was recorded in N2 atmosphere and with a heating rate equal to 10 °C/min.
Figure 10a shows the IR spectra of the emitted gases from the samples collected at 450 °C from the TGA furnace. The main gases observed were CO2 (at 2250 to 2400 cm-1), benzene (at 3080 cm-1 and 740 cm-1), methane (CH4, at 2900 cm-1) together with other carbon-hydrogen molecular fractions such as C2H4, C2H2 and C4H10 (between 2800 and 3100 cm-1 and at 1500 cm-1) and O-H vibration (3500 to 3800 cm-1 and 1600 cm-1). It has been previously reported that the strength of the C-OH bond of the aliphatic bridge in phenolic resins decreases with increasing temperature (Lin and Ma 2000), and this gives places for cleavage of the methylene bridges during the pyrolysis of the samples. As the temperature increases, the ratio of the concentration of the diphenyl ether links to that of the phenol structures increases (Trick and Saliba 1995; Grenier-Loustalot et al. 1996). The diphenyl ether link structures constitute the intermediate structures that are generated from the phenolic resin during the process of thermal degradation, which are followed by the formation of cresol, xylenol, and/or trimethyl-phenol species as the pyrolysis process advances (Jones 1983; Trick and Saliba 1995; Grenier-Loustalot et al. 1996).
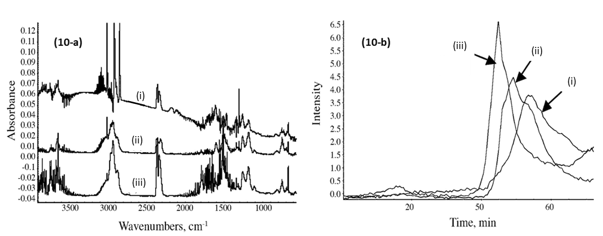
Fig. 10. (a) Infrared spectra of the resin gases emitted at 450 °C from the PF resin sample (i), the CPF 20% cardanol (ii), and with 40% cardanol (iii). (b) Gram-Schmidt plots of the emitted CO2 gas from the PF resin sample (i), the CPF resins 20% cardanol (ii), and with 40% cardanol (iii)
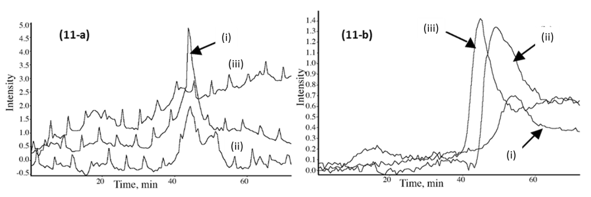
Fig. 11. (a) Gram-Schmidt plots for the emitted gases in the IR region 2750 to 3200 cm-1 (mix of benzene, CH4, C2H2, and C4H10) from the PF resin sample (i), the CPF 20% cardanol (ii), and 40% cardanol (iii). (b) Gram-Schmidt plots for the emitted carbon-hydrogen (CH2 rocking) gas in the IR region 1150-1280 cm-1 from the PF resin sample (i), the CPF 20% cardanol (ii), and 40% cardanol (iii).
The Gram-Schmidt plots were obtained with the OMNICTM Mercury TGA real-time Master software incorporated into the TGA-FTIR system, and they show the intensity of the IR signal of the different gas emissions of the TGA furnace, vs. time of detection (which translates to temperature of the TGA with the heating rate of 10 °C/min). The relative emission of CO gas from the PF sample was lower and at higher temperature than for the CPF resin samples, as shown in Fig. 10b. Similarly, the emissions of aliphatic carbon-hydrogen molecular fractions (-CH2 units), methane, C2H2 and C4H10 compound gases were also lower for the neat PF resin, in comparison with the 20% and 40% cardanol-based resins, as shown in Figs. 11a and 11b. This indicated that the thermal degradation and pyrolysis of the neat PF resin samples were characterized by the formation and emission of relatively fewer gases than in the cardanol-modified CPF resins, in which CO2, methane, and other carbon-hydrogen molecular gas fractions were emitted. The results also showed that the emissions during the pyrolysis of the phenolic carbon-hydrogen molecular fractions increased with the cardanol content in the CPF resins. This indicated that most of the ethylene, methane, and other carbon-hydrogen molecular species emitted were formed by the thermally induced scission and fractionation of the side aliphatic chain of the cardanol, while the formation of the CO2 gas was mostly associated with the breaking of the aromatic ring during the thermal induced degradation of the phenolic samples.
The TGA thermographs in Fig. 12 show that the char yields and the temperature of degradation (Td) went from 58 % and 470 °C for the neat PF resin, to 21% and 400 °C for the CPF resin with 40% cardanol, indicating that the thermal stability and char formation of the CPF resins decreased with the cardanol content. This corresponding decrease in the char formation and cardanol content in the CPF resins resulted in an increase in the volume and quantity of emitted gases during the thermal degradation of the CPF samples (shown previously in the Gram-Schmidt plots in Figs. 10 and 11). Overall, the results from the simultaneous TGA-FTIR analysis revealed that the thermal stability of the cardanol-phenolic samples decreased with the cardanol content while the heat-induced molecular fractionation and gas emissions increased. In general, further research work is required to fully investigate the effect of high concentrations of formaldehyde during the resin synthesis process in the thermal stability and the ultimate properties of the cardanol-based resole phenolic resins.
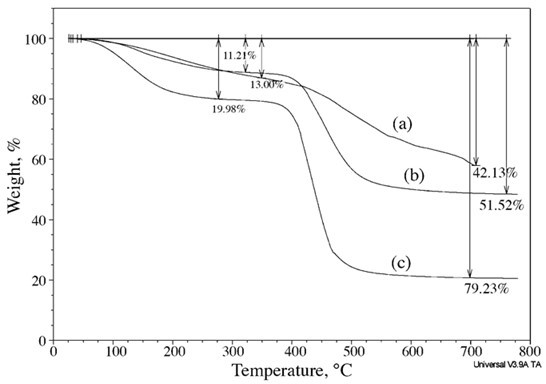
Fig. 12. TGA thermographs of the PF resin (a), the CPF resins with 20% cardanol (b), and CPF resins with 40% cardanol (c), taken during the simultaneous FTIR-TGA analysis. The thermographs were recorded in N2 gas atmosphere and with a heating rate of 10 °C/min.
SEM images (5K) of the tannin-CPF resins showed that the tannin added to the CPF formulations was not fully dissolved in the acid-glycerol solution, as some tannin particles remain uniformly dispersed into the phenolic matrix (Fig. 13).
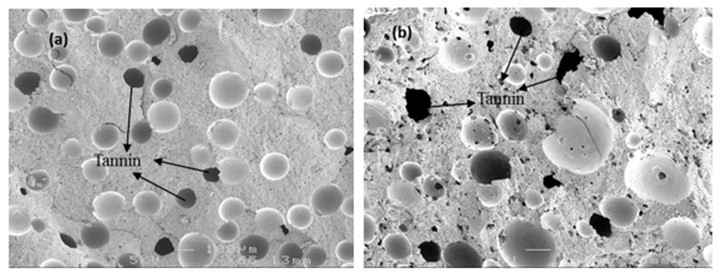
Fig. 13. SEM micrographs of the tannin-CPF cured resins with (a) 20% tannin, and (b) with 40% tannin content (scale 100 mm)
The dissolution of condensed tannins has been previously reported from the bark of black wattle with phenol, in the presence of HCl or H2SO4 acids as catalysts (4% to 5% by weight) and at 160 oC for 90 min (Pizzi 1983; Samil et al. 2005). Even in those conditions, the tannins dissolved to a maximum of only 85% of its initial amount and mostly reacted with the phenol, consuming 77% of the phenol content in the acidic solution. Therefore, it was not surprising that tannin remained partially undissolved and appeared in the SEM micrographs. This uniform dispersion of condensed tannin particles inside of the phenolic cured network, together with the chemical compatibility of tannin and CPF resins, explained the improvement in the flexural properties with the increase of the tannin content in the phenolic resins.
CONCLUSIONS
The thermal stability, as well as the viscoelastic and mechanical properties of cardanol-modified resole phenolic resins blended with different amounts of partially dissolved wattle tannin were investigated. The following conclusions were drawn:
- The best results were obtained with the formulation of P:F molar ratio equal to 1:1.25 and with 40 wt % cardanol substitution of phenol. The resins prepared with higher P:F molar ratio (>1.25) presented a relatively larger variability in their results, which clearly was associated with the high amount of formaldehyde used in the synthesis of the CPF resins.
- The properties of the tannin-CPF resins were proportionally dependent upon the quantity of condensed tannin added, with the natural additive acting as a plasticizer and as a toughening agent to the commonly brittle phenolic resins.
- The solid content and the viscosity of the CPF resin increased with the amount of tannin added to the resins.
- The fracture toughness and the overall flexibility of the synthesized CPF resins increased with the amount of added tannin. The SEM micrographs confirmed that the tannin was only partially dissolved in the acid-glycerol solutions, not unlike a previous report of tannin dissolved in acid-phenol solutions.
- The DMA analysis of the solid post-cured resins revealed that the crosslink density of the tannin-CPF resins have a direct relationship with the tannin content of the resins and its value decreased with increasing tannin content. This effect was due to the flexibility enhancement that the introduction of the polyphenol type of chemical structure of wattle tannin generated inside of the resole resin molecular network.
- The enhanced flexibility of the Tannin-CPF resins compared with the CPF resin was further confirmed by the results of the flexural and fracture toughness tests. The tannin-CPF resins showed a proportional decrease in the flexural modulus with the amount of tannin present in the resins. However, an increase in the flexural strength up to about 20% of added tannin to the CPF resin was observed. Higher tannin content in the CPF resins decreased the flexural strength. Significantly, the fracture toughness values increased for all the tannin-CPF resin formulations, compared with the equivalent values of the neat CPF resins.
- The simultaneous TGA-FTIR analysis revealed that the thermal stability of the cardanol-phenolic samples decreased with the cardanol content while at the same time the heat-induced molecular fractionation and gas emissions of the samples increased.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Ministry of Higher Education, FRGS Grant, No: 5524499 and also to Universiti Putra Malaysia.
REFERENCES CITED
Attanasi, O. A., Buratti, S. B., and Filippone, P. (1995). “Regio-selective bromination of cardanol derivatives,” Org. Prep. Proced. Int. 27(6), 645-712. DOI: 10.1080/00304949509458522
Attanasi, O. A., Filippone, P., and Buratti, S. (1996). “Cardanol: A versatile natural fine chemical largely available today,” La Chimica e l’Industria 78(6), 693-696.
Backeland, L. H. (1909). US Patent Nos 942699, 942700 and 947809.
Balachandran, V. S., Jadhav, S. R., Vemula, P. K., and John, G. (2013). “Recent advances in cardanol chemistry in a nutshell: From a nut to nanomaterials,” Chem. Soc. Rev. 42(2), 427-438. DOI: 10.1039/C2CS35344J
Bloise, E., Becerra-Herrera, M., Mele, G., Sayago, A., Carbone, L., D’Accolti, L., Mazetto, S. E., and Vasapollo, G. (2014). “Sustainable preparation of cardanol-based nanocarriers with embedded natural phenolic compounds,” ACS Sustainable Chem. Eng. 2(5), 1299-1304. DOI: 10.1021/sc500123r
Cardona, F., and Moscou, C. (2009). “Synthesis and characterization of modified phenolic resins for composites with enhanced mechanical properties,” ACMSM20 Conference: Futures in Mechanics of Structures and Materials, 2009, Toowoomba, Australia; http://eprints.usq.edu.au/6515/2/ACMSM20-CardonaF.pdf
Chow, P., Nakayama, F. S., Blahnik, B., Youngquist, J. A., and Coffelt, T. A. (2008). “Chemical constituents and physical properties of guayule wood and bark,” Ind. Crops Prod. 28(3), 303-308. DOI: 10.1016/j.indcrop.2008.03.006
Dharmarajan, N., and Vipulanandan, C. (1988). “Critical stress intensity factor of epoxy mortar,” Polym. Eng. Sci. 28(18), 1182-1191. DOI: 10.1002/pen.760281808 DOI: 10.1016/S0040-4020(02)01025-6
Feng, S., Cheng, S., Yuan, Z., Leitch, M., and Xu, C. (2013). “Valorization of bark for chemicals and materials: A review,” Renewable Sustainable Energy Rev. 26, 560-578. DOI: 10.1016/j.rser.2013.06.024
Filippone, P., Neri, V., Mincione, E., and Saladino, R. (2002). “Selective oxidation of phenol and anisole derivatives to quinones with hydrogen peroxide and polymer-supported methylrhenium trioxide systems,” Tetrahedron 58(42), 8493-8500.
Galia, M., Svec, F., and Frechet, J. M. (1994). “Monodisperse polymer beads as packing material for high-performance liquid chromotography: Effect of divinylbenzene content on the porous and chromatographic properties of poly(styrene-co-divinylbenzene) beads prepared in presence of linear polystyrene as a porogen,” J. Polym. Sci. Pt. A: Polym. Chem. 32 (11), 2169-2175. DOI: 10.1002/pola.1994.080321120
Grenier-Loustalot, M.F., Larroque, S., Grenier, P., and Bedel, D. (1996). “Phenolic resins: 4. Self-condensation of methylolphenols in formaldehyde-free media,” Polym. 37(6), 955-964. DOI: 10.1016/0032-3861(96)87277-6
Gronski, W., Hoffmann, U., Simon, G., Wutzler, A., and Straub, E. (1992). “Structure and density of crosslinks in natural-rubber vulcanizates. A combined analysis by NMR spectroscopy, mechanical measurements, and rubber-elastic theory,” Rubber Chem.Technol. 65(1), 63-77. DOI: 10.5254/1.3538608
ISO 178 (2010). “Plastics – Determination of flexural properties,” International Organization for Standardization, Geneva, Switzerland.
ISO 17281 (2002). “Plastics – Test Method for Determination of Fracture Toughness,” International Organization for Standardization, Geneva, Switzerland.
Jones, R. T. (1983). “The condensation of trimethylolphenol,” J. Polym. Sci.: Polym. Chem. 21(6), 1801-1817. DOI: 10.1002/pol.1983.170210621
Kim, K. H., Tucker, M., and Nguyen, Q. (2005). “Conversion of bark-rich biomass mixture into fermentable sugar by two-stage dilute acid-catalyzed hydrolysis,” Bioresour. Technol. 96(11), 1249-55. DOI: 10.1016/j.biortech.2004.10.017
King, P. W., Mitchell, R. H., and Westwood, A. R. (1974). “Structural analysis of phenolic resole resins,” J. Appl. Polym. Sci. 18(4), 1117-1130. DOI: 10.1002/app.1974.070180412
Knop, A., and Pilato, L. A. (1985). Phenolic Resins: Chemistry, Applications and Performance Future Directions, Springer-Verlag, Berlin, 91-102. DOI: 10.1007/3-662-02429-4
Lee, W. J., and Lan, W. C. (2006). “Properties of resorcinol–tannin–formaldehyde copolymer resins prepared from the bark extracts of Taiwan acacia and China fir,” Bioresour. Technol. 97(2) 257-264. DOI: 10.1016/j.biortech.2005.02.009
Liiri, O., Sairanen, H., Kilpeläinen, H., and Kivistö, A. (1982). “Bark extractives from spruce as constituents of plywood bonding agents,” Holz als Roh- und Werkstoff 40(2), 51-60. DOI: 10.1007/BF02612222
Lin, J. M., and Ma, C. C. M. (2000). “Thermal degradation of phenolic resin/silica hybrid ceramers,” Polym. Degrad. Stab. 69(2), 229-235. DOI: 10.1016/S0141-3910(00)00068-9
Hon, D. N. S., and Shiraishi, N. (2000). Wood and Cellulosic Chemistry. Chemistry of bark, 243–274. 2nd Edition, Marcel Dekker, New York Basel, ISBN 0-8247-0024-4.
Mele, G., Del Sole, R., Vasapollo, G., Garcìa-Lòpez, E., Palmisano, L., Mazzetto, S. E., Attanasi, O. A., and Fillipone, P. (2004). “Polycrystalline TiO2 impregnated with cardanol-based porphyrins for the photocatalytic degradation of 4-nitrophenol,” Green Chem. 6(12), 604-608. DOI: 10.1039/B409510C.
Mottram, J. T., Geary, B., and Taylor, R. (1992). “Thermal expansion of phenolic-fibre composites,” J. Mater. Sci. 27(18), 5015-5026. DOI: 10.1007/BF01105268
Pappa, A., Tzamtzis, N., Statheropoulos, M., and Fasseas, C. (2000). “The pyrolytic behavior of Pinus halepensis needles observed by transmission light microscopy and stereoscopy,” J. Anal. Appl. Pyrolysis 55(2), 195-202. DOI: 10.1016/S0165-2370(99)00099-6
Pizzi, A. (ed.) (1983). Wood Adhesives—Chemistry and Technology, Marcel Dekker, New York. DOI: 10.1163/156856187X00184
Pizzi, A., and Merlin, M. (1981). “A new class of tannins adhesives for exterior particleboard,” Int. J. Adhes. Adhes. 1(5), 261-264. DOI: 10.1016/0143-7496(81)90075-0
Pizzi, A., Moubarika, A., Allal, A., Charriera, F., and Charriera B. (2009). “Cornstarch and tannin in phenol–formaldehyde resins for plywood production,” Ind. Crops and Prod. 30(2), 188–193. DOI: 10.1016/j.indcrop.2009.03.005
Pizzi, Antonio (1982). “Condensed tannins for adhesives,” Ind. Eng. Chem. Prod. Res. Dev 21(3), 359-369. DOI: 10.1021/i300007a005
Pohjamo, S. P., Hemming, J. E., Willför, S. M., Reunanen, M. H. T., and Holmbom, B. R. (2003). “Phenolic extractives in Salix caprea wood and knots,” Phytochemistry 63(2), 165-169. DOI: 10.1016/S0031-9422(03)00050-5
Samil, A., Alma, M. H., and Acemioğlu, B. (2005). “Dissolution of condensed tannin with phenol: Optimum reaction parameters and some flow properties,” J. Appl. Polym. Sci. 98(6), 2450-2453. DOI: 10.1002/app.22441
Seena, J., Sreekalab, M. S., Oommena, Z., Koshy, P., and Sabu, T. (2002). “A comparison of the mechanical properties of phenol formaldehyde composites reinforced with banana fibres and glass fibres,” Compos. Sci. and Technol. 62(14), 1857-1868. DOI: 10.1016/S0266-3538(02)-00098-2
Shafizadeh, J. E., Guionnet, S., Tillman, M. S., and Seferis, J. C. (1999). “Synthesis and characterization of phenolic resole resins for composite applications,” J. Appl. Polym. Sci. 73(4), 505-514. DOI: 10.1002/(SICI)1097-4628(19990725)73:4<505::AID-PP6>3.0.CO;2-L
So, S., and Rudin, A. (1990). “Effects of resin and curing parameters on the degree of cure of resole phenolic resins and woodflour composites,” J. Appl. Polym. Sci. 40(11-12), 2135-2149, 5-20. DOI: 10.1002/app.1990.070401126
Sumin, K., and Hyun-Joong, K. (2003). “Curing behavior and viscoelastic properties of pine and wattle tannin-based adhesives studied by dynamic mechanical thermal analysis and FT-IR-ATR spectroscopy,” J. of Adhes. Sci. and Tech. 17(10), 1369-1383. DOI: 156856103769172797
Torget, R., Himmel, M. E., and Grohmann, K. (1991). “Dilute sulphuric acid pretreatment of hardwood bark,” Bioresour. Technol. 35(3), 239-246. DOI: 10.1016/0960-8524(91)90120-9
Trick, K., and Saliba, T. E. (1995). “Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite,” Carbon 33(11), 1509-1515. DOI: 10.1016/0008-6223(95)00092-R
Usia, M., and Kara, S. (1997). “The chemical composition of wood and bark of Cedrus libani A. Rich,” Holz als Roh- und Werkstoff 55(2), 268. DOI: 10.1007/BF02990561
Vázquez, G., Antorrena, G., González, J. and Alvarez, J. C. (1996). “Tannin-based adhesives for bonding high-moisture Eucalyptus veneers: Influence of tannin extraction and press conditions,” Holz als Roh- und Werkstoff 54(2), 93-97. DOI: 10.1007/s001070050144
Vázquez, G., González-Alvarez, J., Freire, S., López-Suevos, F., and Antorrena, G. (2001). “Characteristics of Pinus pinaster bark extracts obtained under various extraction conditions,” Holz als Roh- und Werkstoff 59(6), 451-456. DOI: 10.1007/s00107-001-0246-0
Wang, S., Adanur, S., and Jang, B. Z. (1997). “Mechanical and thermo-mechanical failure mechanism analysis of fiber/filler reinforced phenolic matrix composites,” Compos. Part B: Eng. 28(3), 215-231. DOI: /10.1016/S1359-8368(96)00042-X.
Yosibash, Z., Bussiba A. and Gilad, I., (2004) Failure criteria for brittle elastic materials, International Journal of Fracture, 125: 307–333. DOI: 10.1023/B:FRAC.0000022244.31825.3b
Article submitted: September 7, 2015; Peer review completed: November 22, 2015; Revised version received and accepted: November 24, 2015; Published: December 3, 2015.
DOI: 10.15376/biores.11.1.965-983
