Abstract
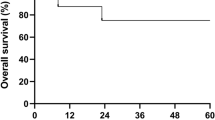
An 18-year-old patient with chronic granulomatous disease who had had at least 2 episodes of life-threatening Aspergillus pneumonia was treated with nonmyeloablative allogeneic stem cell transplantation (NSCT) from an HLA-identical and major ABO-incompatible sibling. The conditioning regimen consisted of cyclophosphamide at a dose of 60 mg/kg (days -5, -4) and fludarabine at a dose of 30 mg/m2 (days -5, -4, -3, -2, -1). Full donor T-cell engraftment was attained on day 28, and full myeloid engraftment was established by day 150 after tacrolimus withdrawal.The bacteriocidal activity of neutrophils, as indicated by flow cytometry with the use of a dichlorofluorescein diacetate oxidation assay, remained low until 150 days after transplantation, but no infection was detected, a finding that suggests mixed chimerism of granulocytes controlled infection. Graftversus-host disease and severe regimen-related toxicity (grade 3 or greater) were not observed. This patient developed prolonged pure red cell aplasia, possibly caused by persistent antidonor isohemagglutinin produced by the residual host B-cells. The aplasia resolved with the combination of erythropoietin, double filtration plasmapheresis, and rituximab. In the setting of major ABO-incompatible NSCT, a fludarabine- and cyclophosphamide-based conditioning regimen may lead to prolonged PRCA.
Similar content being viewed by others
References
Margolis DM, Melnick DA, Alling DW, Gallin JI. Trimethoprimsulfamethoxazole prophylaxis in the management of chronic granulomatous disease. J Infect Dis. 1990;162:723–726.
Mouy R,Veber F, Blanche S, et al. Long-term itraconazole prophylaxis against Aspergillus infections in thirty-two patients with chronic granulomatous disease. J Pediatr. 1994;125:998–1003.
International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Engl J Med. 1991;324:509–516.
Cohen MS, Isturiz RE, Malech HL, et al. Fungal infection in chronic granulomatous disease. Am J Med. 1981;71:59–62.
Winkelstein JA, Marino MC, Johnston RB, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine. 2000;79:155–169.
Seger RA, Gungor T, Belohradsky BH, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hematopoietic allograft: a survey of the European experience 1985–2000. Blood. 2002;100:4344–4350.
Leung TF, Chik KW, Li CK, Shing MMK, Yuen PMP. Bone marrow transplantation for chronic granulomatous disease: long-term follow-up and review of literature. Bone Marrow Transplant. 1999; 24:567–570.
Horwitz ME, Barrett J, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning regimen and a T-cell-depleted hematopoietic allograft. N Engl J Med. 2001;344:881–888.
Gmur JP, Burger J, Schaffner A, et al. Pure red cell aplasia of long duration complicating major ABO-incompatible bone marrow transplantation. Blood. 1990;75:290–295.
Bolan CD, Leitman SF, Griffth LM, et al. Delayed donor red cell chimerism and pure red cell aplasia following major ABO-incompatible nonmyeloablative hematopoietic stem cell transplantation. Blood. 2001;98:1687–1694.
Gregg JS, Stuart W. Rituximab therapy and autoimmune diseases. Arthritis Rheum. 2003;48:1484–1492.
Ariga T, Sakiyama Y,Tomizawa K, et al. A newly recognized point mutation in the cytochrome b558 heavy chain gene replacing alanine 57 by glutamic acid in a patient with cytochrome b positive X-linked chronic granulomatous disease. Eur J Pediatr. 1993;152: 469–472.
Thiede C, Florek M, Bornhauser M, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999;23:1055–1060.
Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241.
Morishima Y, Morishita Y, Tanimoto M, et al. Low incidence of acute graft-versus-host disease by the administration of methotrexate and cyclosporine in Japanese leukemia patients after bone marrow transplantation from human leukocyte compatible siblings: possible role of genetic homogeneity. The Nagoya Bone Marrow Transplantation Group. Blood. 1989;74:2252–2256.
Maciej Zaucha J, Mielcarek M, Takatu A, et al. Engraftment of early erythroid progenitors is not delayed after non-myeloablative major ABO-incompatible haematopoietic stem cell transplantation. Br J Haematol. 2002;119:740–750.
Peggs KS, Morris EC,Kottaridis PD, et al. Outcome of major ABO incompatible nonmyeloablative hematopoietic stem cell transplantation may be influenced by conditioning regimen. Blood. 2002;99: 4642–4644.
Ohta S,Yokoyama H, Ise T, et al. Apheresis therapy for prolonged red cell aplasia after major ABO-mismatched bone marrow transplantation. Intern Med. 1997;36:487–491.
Santamaria A, Sureda A, Martino R, Domingo-Albos A, Muniz- Diaz E, Brunet S. Successful treatment of pure red cell aplasia after major ABO-incompatible T cell-depleted bone marrow transplantation with erythropoietin. Bone MarrowTransplant. 1997;20:1105–1107.
Maloney BD, Grillo-Lopez AJ, White CA, et al. IDEC-C288 (rituximab) Anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade Non-Hodgkin’s lymphoma. Blood. 1997; 90:2188–2195.
Maschan AA, Skorobogatova EV, Balashov DN, et al. Successful treatment of pure red cell aplasia with a single dose of rituximab in a child after major ABO incompatible peripheral blood allogeneic stem cell transplantation for acquired aplastic anemia. Bone Marrow Transplant. 2002;30:405–407.
Stasi R, Pagano A, Stipa E, Amadori S. Rituximab chimeric anti- CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood. 2001;98:952–957.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fujiwara, T., Yamada, M., Miyamura, K. et al. Fludarabine- and Cyclophosphamide-Based Nonmyeloablative Conditioning Regimen for Transplantation of Chronic Granulomatous Disease: Possible Correlation with Prolonged Pure Red Cell Aplasia. Int J Hematol 79, 293–297 (2004). https://doi.org/10.1532/IJH97.03123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1532/IJH97.03123