Abstract
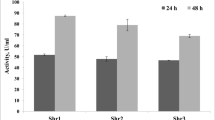
Among various strains of Bacillus subtilis, the strain RNZ-79 was selected for this study due to the highest uricase productivity in solid state cultures containing shrimp shell wastes, highest Vmax (0.42 μM mg−1min−1) and lowest value of Km (56 μM). Maximum productivity was observed at pH 7.6 and 45°C with an inducer concentration of 0.4% (w/v) uric acid and moisture content of 80% (v/w). An inoculum of 7% (v/v) and particle size of 354–500 μm for substrate were optimal for productivity after 54 h of fermentation. None of the tested carbon and inorganic nitrogen sources had stimulatory effect on uricase productivity. KH2PO4 at 0.12% (w/v) was the best source of phosphorus. Final uricase productivity was 5.05-fold more than the initial productivity. Enzyme purification increased the specific activity to 25-fold with a recovery of 36%. Furthermore, the purified enzyme showed a molecular mass of 33.7 kDa. Purified uricase was optimally active at pH 8.0 and 40°C and maximally stable at pH 7.0–10.0 and till 70°C for 30 min. The half-life (t1/2) at 60, 70, 80, 90 and 100°C were 87.0, 56.6, 30.0, 24.2 and 15.6 min, respectively, and the calculated midpoint temperature (Tm) was 66.85°C. Interestingly, purified enzyme exhibited a good storage stability for 3 months and none of uric acid analogues were competitive inhibitor, indicating a high specificity of uricase.
Similar content being viewed by others
Abbreviations
- CB:
-
corn bran
- LSCs:
-
liquid state cultures
- LSF:
-
liquid state fermentation
- RB:
-
rice bran
- SBP:
-
soybean powder
- SSCs:
-
solid state cultures
- SSF:
-
solid state fermentation
- SSP:
-
shrimp shell powder
- WB:
-
wheat bran
References
Abd El Fattah Y., Saeed H., Gohar Y. & El-Baz M. 2005. Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process Biochem. 40: 1707–1714.
Abdel-Fattah G. & Abo-Hamed N. 2002. Bioconversion of poultry wastes I-factors influencing the assay and productivity of crude uricase by three uricolytic filamentous fungi. New Microbiol. 25: 57–64.
Adámek V., Králová B., Suchová M., Valentová O. & Demnerová K. 1989. Purification of microbial uricase. J. Chromatogr. 497: 268–275.
Adámek V., Suchová M., Demnerová K., Králová B., Fořt I. & Morava P. 1990. Fermentation of Candida utilis for uricase production. J. Ind. Microbiol. 6: 85–90.
Alamillo J., Cardenas J. & Pineda M. 1991. Purification and molecular properties of urate oxidase from Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1076: 20–208.
Anderson A. & Vijayakumar S. 2011. Purification and optimization of uricase enzyme produced by Pseudomonas aeruginosa. J. Exp. Sci. 2: 5–8.
Bailey W., Scott E., Finegold S. & Baron E. 1986. Bailey and Scott’s Diagnostic Microbiology. Mosby Co., USA.
Baysal Z., Uyar F. & Aytekin C. 2003. Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem. 38: 1665–1668.
Bongaerts G., Uitzetter J., Brouns R. & Vogels G. 1978. Uricase of Bacillus fastidiosus. Properties and regulation of synthesis. Biochim. Biophys. Acta 527: 348–358.
Brandenburg J., Wray L., Beier L., Jarmer H., Saxild H. & Fisher S. 2002. Roles of PucR, GInR, and TnrA in regulating expression of the Bacillus subtilis ure P3 promoter. J. Bacteriol. 84: 6060–6064.
Hassanein W., Kotb E., Awny N. & El-Zawahry Y. 2011. Fibrinolysis and anticoagulant potential of a metallo protease produced by Bacillus subtilis K42. J. Biosci. 36: 77–779.
Hibi T., Hayashi Y., Fukada H., Itoh T., Nago T. & Nishiya Y. 2014. Intersubunit salt bridges with a sulfate anion control subunit dissociation and thermal stabilization of Bacillus sp. TB-90 urate oxidase. Biochemistry 53: 3879–3888.
Huang S. & Wu T. 2004. Modified colorimetric assay for uricase activity and a screen for mutant Bacillus subtilis uricase genes following StEP mutagenesis. Eur. J. Biochem. 271: 517–523.
Ishikawa J., Yamashita A., Mikami Y., Hoshino Y., Kurita H., Hotta K., Shiba T. & Hattori M. 2004. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. USA 101: 14925–14930.
Kai L., Ma X., Zhou X., Jia X., Li X. & Guo K. 2008. Purification and characterization of a thermostable uricase from Microbacterium sp. strain ZZJ4-1. World J. Microbiol. Biotechnol. 24: 401–406.
Kotb E. 2015a. Characterization of a thermostable uricase isolated from Bacillus firmus DWD-33 and its application for uric acid quantification in human serum. Protein Pept. Lett. 22: 402–409.
Kotb E. 2015b. The biotechnological potential of subtilisin-like fibrinolytic enzyme from a newly isolated Lactobacillus plantarum KSK-II in blood destaining and antimicrobials. Biotechnol. Prog. 31: 316–324.
Koyama Y., Ichikawa T. & Nakano E. 1996. Cloning, sequence analysis, and expression in Escherichia coli of the gene encoding the Candida utilis urate oxidase (uricase). J. Biochem. 120: 969–973.
Krishna C. & Chandrasekaran M. 1996. Banana waste as substrate for a-amylase production by Bacillus subtilis (CBTK 106) under solid-state fermentation. Appl. Microbiol. Biotechnol. 46: 106–111.
Laemmli U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Liu J., Li G., Liu H. & Zhou X. 1994. Purification and properties of uricase from Candida sp. and its application in uric acid analysis in serum. Appl. Biochem. Biotechnol. 47: 57–63.
Lonsane B., Saucedo-Castaneda G., Raimbault M., Roussos S., Viniegra-Gonzalez G., Ghildyal N., Ramakrishna M. & Krishnaiah M. 1992. Scale-up strategies for solid state fermentation systems. Process Biochem. 27: 259–273.
Lotfy W. 2008. Production of a thermostable uricase by a novel Bacillus thermocatenulatus strain. Bioresour. Technol. 99: 699–702.
Lowry O., Rosebrough N., Farr A. & Randall R. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Montalbini P., Aguilar M. & Ineda M. 1999. Isolation and characterization of uricase from bean leaves and its comparison with uredospore enzyme. Plant Sci. 147: 139–147.
Nakagawa T., Mazzali M., Kang D., Sanchez-Lozada L., Herrera-Acosta J. & Johnson R. 2006. Uric acid - a uremic toxin? Blood Purif. 24: 67–70.
Nanda P. & Babu P. 2014. Isolation, screening and production studies of uricase producing bacteria from poultry sources. Prep. Biochem. Biotechnol. 44: 811–821.
Pandey A., Selvakumar P., Soccol C. & Nigam P. 1999. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 77: 149–162.
Pandey A., Soccol C. & Larroche C. 2007. Current Developments in Solid-State Fermentation. Springer Science/Asiatech Publishers, New York, USA/New Delhi, India.
Pfrimer P., de Moraes L., Galdino A., Salles L., Reis V., De Marco J., Prates M., Bloch C. & Torres F. 2010. Cloning, purification, and partial characterization of Bacillus subtilis urate oxidase expressed in Escherichia coli. Biomed. Biotechnol. 2010: 1–6.
Saeed H., Abdel-Fattah Y., Gohar Y. & Elbaz M. 2004. Purification and characterization of extracellular Pseudomonas aeruginosa urate oxidase enzyme. Pol. J. Microb. 53: 45–52.
Schiavon O., Caliceti P., Ferruti P. & Veronese F. 2000. Therapeutic proteins: a comparison of chemical and biological properties of uricase conjugated to linear or branched poly (ethylene glycol) and poly(N-acryloylmorpholine). Farmaco 55: 264–269.
Schultz C., Nygaard P. & Saxild H. 2001. Functional analysis of 14 genes that constitute the purine catabolic pathway in Bacillus subtilis and evidence for a novel regulon controlled by the PucR transcription activator. J. Bacteriol. 183: 329–3302.
Suzuki K., Sakasegawa, S., Misaki H. & Sugiyamac M. 2004. Molecular cloning and expression of uricase gene from Arthrobacter globiformis in Escherichia coli and characterization of the gene product. J. Biosci. Bioeng. 98: 15–158.
Swan D., Hale R., Dhillon N. & Leadlay P. 1987. A bacterial calcium-binding protein homologous to calmodulin. Nature 329: 84–85.
Tanaka A., Yamamura M., Kawamoto S. & Fukui S. 1977. Production of uricase by Candida tropicalis using n-alkane as substrate. Appl. Environ. Microbiol. 34: 342–346.
Wallrath L. & Friedman T. 1991. Species differences in the temporal pattern of Drosophila urate oxidase gene expression are attributed to transacting regulatory changes. Proc. Natl. Acad. Sci. USA 88: 5489–5493.
Werner A. & Witte C. 2011. The biochemistry of nitrogen mobilization: purine ring catabolism. Trends Plant Sci. 16: 381–387.
Yamamoto K., Kojima Y., Kikuchi T., Shigyo T., Sugihara K., Takashio M. & Emi S. 1996. Nucleotide sequence of the uri-case gene from Bacillus sp. TB-90. J. Biochem. 119: 80–84.
Yazdi M., Zarrini G., Mohit E., Faramarzi M., Setayesh N., Sedighi N. & Mohseni F. 2006. Mucor hiemalis: a new source for uricase production. World J. Microbiol. Biotechnol. 22: 325–330.
Yokoyama S., Ogawa A. & Obayashi A. 1988. Rapid extraction of uricase from Candida utilis cells by use of reducing agent plus surfactant. Enzyme Microb. Technol. 10: 52–55.
Zhao Y., Zhao L., Yang G., Tao J., Bu Y. & Liao F. 2006. Characterization of a uricase from Bacillus fastidious A.T.C.C. 26904 and its application to serum uric acid assay by a patented kinetic uricase method. Biotechnol. Appl. Biochem. 45: 75–78.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kotb, E. Improvement of uricase production from Bacillus subtilis RNZ-79 by solid state fermentation of shrimp shell wastes. Biologia 71, 229–238 (2016). https://doi.org/10.1515/biolog-2016-0040
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2016-0040