Soot: Giver and Taker of Light
By Christopher Shaddix, Timothy Williams
The complex structure of soot greatly influences the optical effects seen in fires
The complex structure of soot greatly influences the optical effects seen in fires

DOI: 10.1511/2007.65.232
From earliest childhood, people are fascinated with the flickering yellow glow of candle flames and burning logs. However, few of us realize, even in adulthood, that soot—a material that epitomizes blackness—is behind that warm light.
The 19th-century physicist Michael Faraday put it well when he said, "You would hardly think that all those substances which fly about London, in the form of soot and blacks, are the very beauty and life of the flame." Indeed, if it weren't for the presence of clouds of tiny soot particles within these fires, they would appear blue, like those on a well-operating gas stove, which give off considerably less visible and thermal radiation.

AP Images/Hertfordshire Police
We can vividly illustrate this phenomenon in the laboratory. Ethylene, for example, burning in air yields plenty of soot and a bright yellow flame, whereas the same fuel diluted with nitrogen to suppress the formation of soot gives a much dimmer blue flame. The cerulean color arises from the highly excited products of the combustion reaction. These molecules emit light in discrete spectral bands that correspond to the excitation levels of their electrons. One of the primary molecules is of a class called radicals because they have unpaired electrons in their outer shells. This radical is made from a single carbon and a single hydrogen, denoted CH*, which emits light at a wavelength of about 431 nanometers—squarely at the blue end of the visible spectrum.
In contrast, the light emitted from soot extends across the visible wavelength range and into the near-infrared. Cool soot looks black, but while it's in a flame, the heat liberated from the surrounding combustion reactions makes these particles incandesce like so many tiny light-bulb filaments. Gas molecules cannot absorb or emit such large amounts of energy across a range of wavelengths. As Faraday correctly surmised, it is the solid nature of soot that gives most flames their distinctive luminescent qualities.
A combination of factors explains why the radiation from soot typically appears yellow. Part has to do with way the human eye responds to the spectral variation in soot emissivity (the ratio of the light given off to the light radiated by a perfect black body). Another reason has to do with the typical temperature of soot (around 1,400 degrees Celsius). Hotter soot appears whiter and cooler soot will be redder, just as is true for heated metal. Thus soot often provides some pleasing optical effects. But these radiant abilities can also lead accidental fires to grow swiftly out of control. That is, although soot is the offspring of flame, it alters the nature of its creator.
At 6:00 a.m. on Sunday, December 11, 2005, the Buncefield oil-storage depot, located just outside London, erupted in a plume of fire and hot gases, the biggest conflagration that country had ever seen in peacetime. Forty-three people were injured by a series of explosions, and 2,000 were evacuated from the surrounding area. Twenty large fuel-storage tanks burned, and it took firefighters several days to quell the blaze. Meanwhile, huge clouds of black smoke billowed into the skies.
This calamity provided a dramatic demonstration of the complex role of soot in fires. Photographs of the flames emanating from the burning gasoline and aviation fuel showed the bright yellow and orange colors characteristic of hot soot particles. From a distance, however, the flames were dwarfed by the sooty smoke cloud, which blocked out the sun in the local area and could be seen wafting as far as France in satellite images.
Similar catastrophes have struck other fuel depots around the world at different times. And devastating fires have taken place on a smaller scale when fuel-laden planes and trucks have crashed. Some noteworthy incidents in recent years have involved automobile and train tunnels, particularly in the European Alps. In California, just east of San Francisco Bay, a fuel-truck spill in 1982 turned the Caldecott tunnel into an inferno.
The attention given to this class of fires rose after the terrorist attacks of September 11, 2001, in which large volumes of aviation fuel were deposited and burned in the World Trade Center buildings, contributing to their collapse. The oil-well fires during the first Iraqi war were another high-profile example.
The fires at Buncefield were of a type referred to in scientific discussions as "pool fires," meaning that the flames were intimately coupled to pools of liquid fuel. Pool fires are known for the difficulty of extinguishing them, their tendency to form soot and smoke, and their high levels of radiant heat transfer, all in evidence at Buncefield.
In battling such an emergency, responders have to make decisions that, if wrong, can cost their lives. When deciding whether to risk putting fire-fighting personnel anywhere near a pool of burning fuel, it's critical to know how long the walls of adjacent fuel-storage tanks will last when exposed to the intense heat. This calculation is, in part, a radiant heat-transfer issue, which is fundamentally controlled by the production of soot and smoke. In pool fires with hydrocarbon fuels, the thermal radiation from soot dominates heat transfer, but conversely, clouds of cold soot particles in smoke insulate the surroundings from thermal radiation. Hence it is important for scientists to understand how exactly soot forms within fires and becomes smoke.
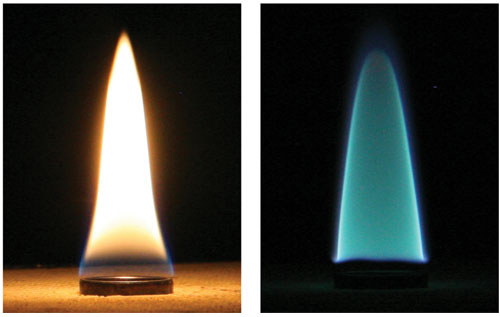
Photographs courtesy of the authors.
Combustion requires fuel, which in the cases of interest is most often made from long, complex chains of carbon and hydrogen atoms. When a flame is lit, the heat breaks apart these hydrocarbons in a process called pyrolysis. The smaller chunks that result are often radicals, highly inclined toward chemical reactions, in particular oxidation: Oxygen combines with the carbon and hydrogen radicals to produce carbon dioxide and water, releasing heat in the process.
However, some of the radicals react with one another, rather than with oxygen, forming rings of carbon called polycyclic aromatic hydrocarbons. These newly formed compounds continue to grow into carbon-rich lattices and then into full-fledged particles, which agglomerate into long chains that resemble strings of beads. As these soot masses travel upward inside a flame, they react with oxygen molecules, which can break off pieces and cause the particles to incandesce more brightly, creating the flame's bright yellow glow. Whether or not the soot will be fully burned in this way (completely transformed into carbon dioxide and water) before leaving the flame depends on the type of fire. If not completely burned, the residual soot is released as smoke.
Tall, buoyant flames with relatively lazy mixing of the fuel and air promote the formation of soot, because there is ample residence time within these flames for fuel molecules to pyrolyze and then recombine. The larger hydrocarbon compounds made in this way eventually come together and form carbonaceous particles.
The thermal radiation given off by this soot is important to sustaining (and initially growing) pool fires. Indeed, the presence of soot allows up to 50 percent of the energy in a flame to be transferred back to the pool of fuel, where it acts to vaporize the liquid and sustain the reaction. In large fires, thermal radiation from the flames provides almost all of the energy transferred to the liquid fuel. Ironically, it is the radiant emission given off by the hot soot that results in the thermal quenching of flames at the top and around the periphery of the fire, leading to the release of the cool, light-absorbing soot. The radiant heat transfer exceeds 100 kilowatts per square meter for pools 2 meters across but is less for larger pools, which tend to produce thick layers of smoke around the fire. (In comparison, solar radiation on a clear, dry day with the sun directly overhead is 1 kilowatt per square meter.)
As important as they are in accidental fires, soot particles also influence people's lives in other ways. Commercial combustion products, called carbon blacks, have long been ingredients in rubbers and inks, and soot was the original source of the soccer-ball-shaped carbon molecules called fullerenes, as well as carbon nanotubes, both of which are of great interest in the field of nanotechnology. Soot plays the benefactor in boilers and furnaces that rely on flame radiation to transfer heat to the walls (to generate steam, for example), but the same mechanism makes these particles harmful for internal-combustion engines, where such heat losses decrease efficiency and require that high-temperature materials be used.
Soot emissions to the atmosphere from industrial smokestacks and automobile tailpipes contribute to the formation of light-absorbing haze and have recently received attention for their influence on climate. This connection arises because the more sunlight that is reflected back out into space, the cooler the Earth becomes, whereas the more sunlight absorbed in the lower atmosphere or on the surface, the more the planet is warmed. Hence the effect of soot particles on climate strongly depends on their optical properties, specifically how they scatter and absorb sunlight.
Most climatologists believe that atmospheric soot enhances global warming, but some studies have suggested that soot can have a cooling effect, too, because these particles can act as nucleation points for molecules of water vapor, allowing more sunlight-reflective clouds to form. Soot also influences climate because it is deposited on snow and ice surfaces, darkening them and thus increasing their rate of melting.
The absorptivity of cold soot is important in determining not only how effectively it takes in light, but also how effectively hot soot emits light, by virtue of a principle called Kirchoff's law: The emissivity of a substance at thermal equilibrium is always equal to its absorptivity. Quantifying the optical properties of soot has proven to be difficult, because it is such a heterogeneous substance. Its atomic bonding is dependent on the environment in which it is formed, thereby affecting its optical properties. (Just think of the contrast in optical properties between diamond and graphite, both pure forms of carbon.) Indeed, the quest for a better understanding of this subject has quite a long history.
An appreciation of soot's optical properties began, appropriately, in London early in the industrial age, when the city was choked with smoke from the widespread burning of coal. It was in this era that Faraday carried out research on flames. He soon understood the importance of the optical properties of soot. Faraday's knowledge of this subject was built on the first extensive studies of combustion initiated by his mentor at the Royal Institution, Sir Humphry Davy, when Davy was developing the coal miner's safety lamp in 1815. Faraday was a popular lecturer, and for many years during the Christmas holidays of the mid-1800s, he gave a lecture series for young audiences titled "The Chemical History of a Candle," in which he demonstrated that a flame without soot produces a very weak glow. Faraday clearly saw the irony: "Is it not beautiful to think that such a process is going on, and that such a dirty thing as charcoal can become so incandescent?"

The Royal Institution, London, UK/The Bridgeman Art Library
After Faraday, the optical properties of soot did not receive much attention until the early 20th century, when combustion-related research began to develop as an active field. A number of German investigators examined the radiation properties of soot in an attempt to determine flame temperature and to understand the contribution these particles made to the transfer of heat in furnaces and boilers.
In the United States in the 1930s, Hoyt C. Hottel, a professor researching heat transfer in furnaces at the Massachusetts Institute of Technology, improved on the optical pyrometer, an instrument much like a thermometer except that it measures temperature without contacting the surface. The device uses a filter to determine the amount of energy being radiated from a sample in a single wavelength, or color, which is compared with a table of known temperature-color relations. Hottel developed the first two-color device for measuring the temperature of soot and demonstrated its superiority to conventional single-color methods. (The advantage is that one can determine temperature from the ratio of the two light intensities, whereas a single-color instrument needs to measure absolute amounts and is thus difficult to calibrate.) Later on, Hottel measured the angular distribution of light scattering by soot inside a flame and in the early 1960s collected some of the first images of soot particles by transmission electron microscopy (TEM), showing a highly agglomerated structure of primary particles about 25 nanometers in diameter.
At about the same time, Roger C. Millikan, a researcher at General Electric, measured the spectral variation of soot absorption above a horizontally flat flame and also analyzed the atomic ratio of hydrogen to carbon in the soot. He found that with increasing height above the burner, this ratio decreased, as did the spectral variation of optical absorption. This observation gave the first concrete evidence that the absorption properties of soot varied with position within a flame and were linked to the chemical composition of the soot.
In 1969, Adel F. Sarofim, a former graduate student of Hottel's who joined him on the faculty of MIT, published the results of measurements of the index of refraction of soot collected from propane- and acetylene-fueled flames. The index of refraction is the Holy Grail of material optical properties, allowing the calculation of both absorption and scattering of various shapes of the material, including round particles of a known size.
The index is a measurement of the path that light will take within a particular material. The value is expressed as a complex number, with the real component relating to the velocity of light within the material relative to the speed of light in vacuum. This component also indicates how much a ray of light will be bent, or refracted, when it passes through the material. The imaginary term indicates how much of the light is lost due to absorption. Sarofim reported the index of refraction of the soot he measured to be 1.57 - 0.56i.
This result was an improvement over past attempts to measure the optical properties of soot, and Sarofim had the convenient result of finding a similar refractive index for both fuel sources and for a range of visible wavelengths. Also, a number of succeeding determinations produced values close to Sarofim's. As a consequence, his value for the refractive index was widely adopted in both combustion- and fire-research communities as the refractive index of soot, and it has largely retained that status all the way to the present.
A few investigators pointed out long ago that the method Sarofim and others had used to determine the index of refraction was subject to significant errors. The technique required soot aggregates to be pressed into a smooth surface, a virtually impossible task to perform with hard, agglomerated nanoparticles. In 1979, Jay Janzen from Phillips Petroleum Company made the prescient observation that not only were there unavoidable errors in this approach, but that the derived refractive-index values were inconsistent with well-established measurements of the amount of light a given volume of soot particles extinguish (through absorption and, to a lesser extent, scattering). Janzen performed measurements of the spectral extinction of small, uniform particles of carbon black and calculated a refractive index that was quite different (2.0 - 1.0i). Unfortunately, this work was published in a journal that was not typically read by combustion and fire researchers, so it went unnoticed.
For several years now, we have been investigating large-scale pool fires, both experimentally and numerically, because of the risk they pose during transport accidents. In the course of developing and validating our computational models, we've acquired a keener appreciation for soot in determining how fires spread. In particular, knowledge of the soot concentration, temperature and optical properties within these fires is required to quantify the amount of heat transferred.
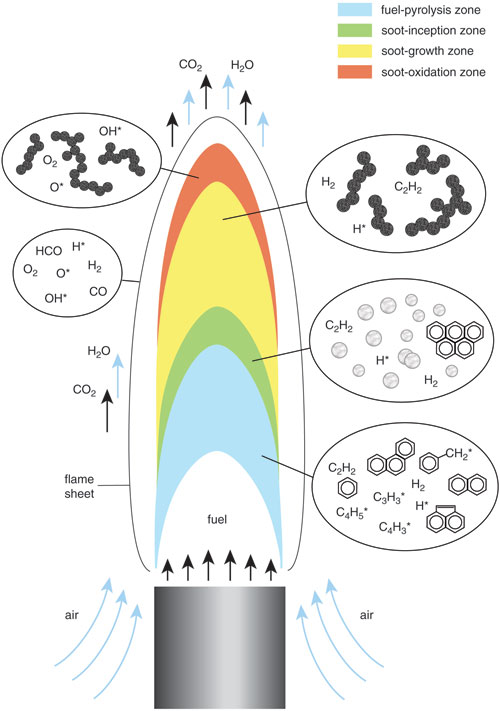
Stephanie Freese
Over the past 15 years, several investigators have shown through TEM and optical measurements that when soot begins to form in a flame, it first develops liquid-like precursor particles, which have little absorptivity. These tarry particles contain a relatively high amount of hydrogen and, unlike the more commonly investigated solid soot particles, they coalesce into a single, larger particle when they collide. Those larger blobs in turn lose some of their hydrogen, and at a critical ratio of hydrogen to carbon of around 0.2, they solidify into hard carbonaceous particles, which agglomerate into clusters upon colliding.
Researchers from the University of Naples have shown that the precursor particles first only absorb light in the ultraviolet region of the spectrum and then, as the polycyclic aromatic hydrocarbons grow in size and lessen the energy required to put the electrons into an excited state, the optical absorptivity extends into visible wavelengths and ultimately into the infrared. At this point, in other words, the soot becomes "black," absorbing all visible wavelengths.
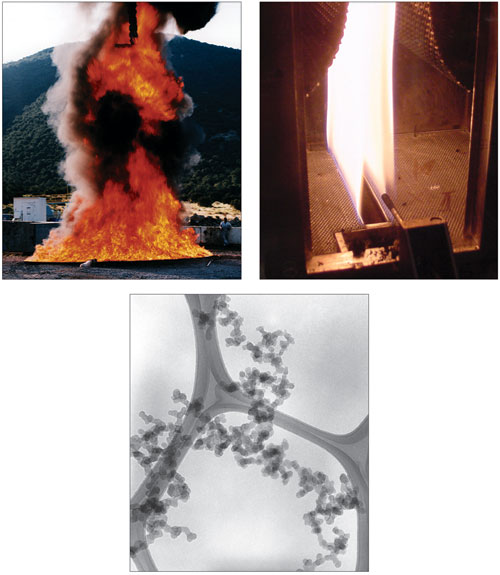
Photographs courtesy of the authors.
One unanswered question was whether the solid carbon particles formed in this way become denser as they travel upward in the flame. This development could lead to variations in the soot nanostructure and thus its optical absorptivity. To investigate the possibility of such soot densification, we worked with collaborators at the University of Illinois at Chicago, at Drexel University and at Sandia National Laboratories to collect soot particles onto grids of loosely packed fibers that can be examined with a microscope. We extracted samples from different heights in steady and unsteady laboratory flames burning ethylene, a simple hydrocarbon fuel gas, and in a large-scale turbulent pool fire burning JP-8, a type of fuel used mostly for powering military jets. We then analyzed these soot samples using high-resolution TEM, which resolves the structure of the resultant particles below the nanometer level.
We were not able to study soot precursors in the same way, because tar components associated with these particles were also deposited on the collection grids. High-resolution TEM exposes the samples to high fluxes of electrons, which caused these tars to spread out over the soot particles, obscuring their structure.
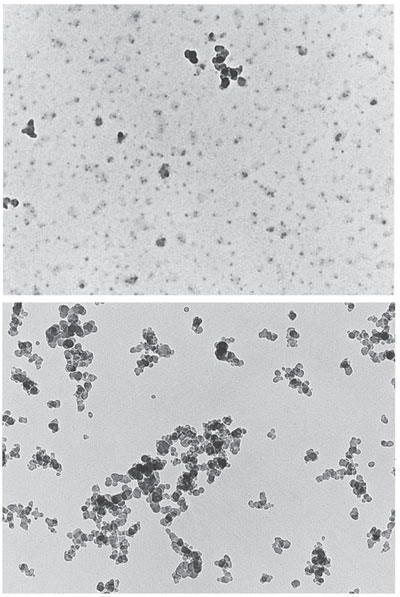
Images courtesy of Richard A. Dobbins, from Physical and Chemical Aspects of Combustion: A Tribute to Irvin Glassman, edited by Frederick L. Dryer and Robert F. Sawyer, published by Taylor & Francis Books.
For mature soot, the TEM images we and other researchers have obtained show that, internally, the particles consist of many thin layers of flat carbon structures arranged in a spiral pattern, vaguely similar to a rolled-up scroll. The particles seem to start at one or several nucleation sites, with growth curling around each of them. The build-up is made from layers of thousands of disordered pieces. Soot thus differs from other forms of carbon, such as fullerenes or graphite, which have highly ordered structures. The presence of hydrogen and occasionally a little oxygen in soot accounts for at least some of this irregularity.
To quantify the characteristics of the carbon layers in our high-resolution TEM images, we enlisted the help of Árpad Palotás of the University of Miskolc in Hungary, who had earlier modified a commercial image-analysis program for this very purpose. Palotás developed this technique while he was a graduate student working under Sarofim. It turns out that the most important characteristic of soot in determining its optical absorptivity is the spacing of adjacent carbon layers, which is an indirect measure of the amount of ordering in the particle. When there is more order, carbon atoms are more tightly bound and lose some of their associated hydrogens. As a result, the carbons become more metal-like, meaning their electrons require less energy to move, so they absorb longer wavelengths of light.
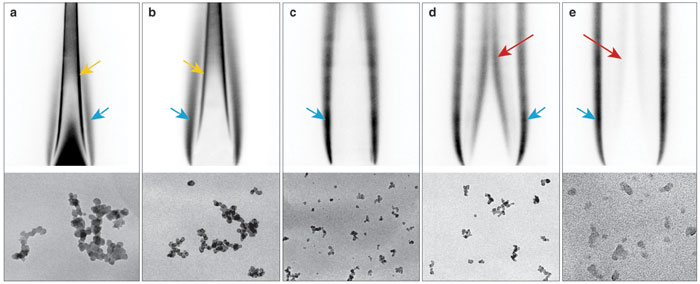
Images courtesy of the authors.
Remarkably, we found that the mean spacing between the carbon layers was nominally the same—from 3.47 to 3.57 angstroms—for all of our soot samples. For comparison, the interlayer spacing of pure graphite, the most ordered form of carbon after diamond and fullerenes, is 3.35 angstroms. The results from this study imply that the index of refraction is essentially the same for soot from different flames, once the sooty material has hardened into solid particles.Thus, historical studies of the variation in soot absorptivity as a function of height in flames can now be understood as reflecting the variable contribution from precursor particles, which become progressively less abundant as one samples higher and higher within a flame. At some height, only solid soot particles are present in the flame or in the emitted smoke, and the absorptivity of the soot stays constant.
The results from our investigation of soot nanostructure begged the long-standing question of just what is the correct value for the index of refraction of solid, carbonaceous soot. To get at the answer, we collaborated with investigators in Sandia's fire-research program to measure extinction coefficients (the exponential factor by which light dims as it passes through a material), from which we could derive values for absorptivity and, in turn, the refractive index. The samples were taken at different heights within laboratory flames burning methane, ethylene and kerosene (a stand-in for jet fuel). Flames used were of the standard variety or were slot flames, where the plume is basically restricted to two dimensions. Slot flames heat the fuel stream more slowly, so soot would be expected to form later and mature less rapidly. The use of these flames let us explore the effect of residence time on soot formation.
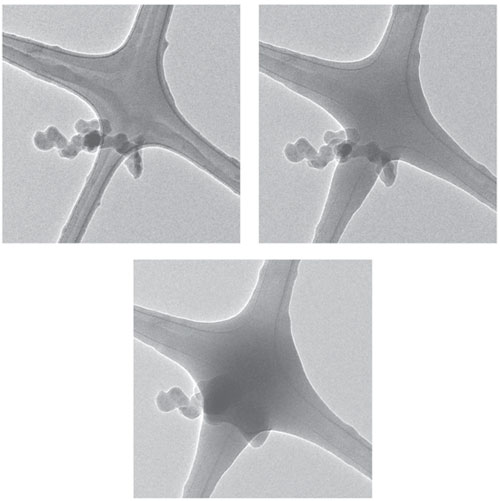
Images courtesy of the authors.
Determining the extinction coefficient entailed extracting soot and the surrounding gases from the flames with a metal tube, capturing the soot-laden gases in a transparent cell where the dimming of a laser beam passing through could be measured, and then collecting the tested soot onto a filter for determination of its mass. To ensure that large hydrocarbon molecules—which might condense into solids in the gas sampling and cooling process—did not influence the measurement of mass, we passed methylene chloride, a common solvent for heavy hydrocarbons, through the soot samples, which were then placed in a vacuum (to remove the methylene chloride and any hydrocarbons present) before they were dried and reweighed.
For the soot derived from ethylene and kerosene flames, we found values for the extinction coefficient that were consistent with measurements made by other investigators of soot emitted from the tops of flames. For the soot extracted from a methane flame, however, we found a markedly lower value.
We surmised that this disagreement may be a consequence of a difference in particle size, which would affect the amount of light scattered. Individual soot particles are usually too small to produce significant amounts of scattering, but as these particles aggregate, they can grow large enough to scatter as much as 30 percent of the amount of light they absorb.
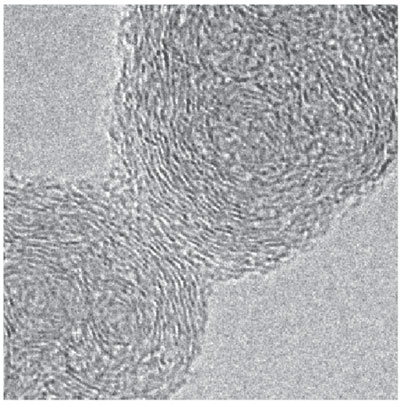
Image courtesy of the authors
Further measurements showed that aggregated soot particles derived from the burning of ethylene and kerosene are significantly larger than those produced by methane-fueled flames. Methane, having a simpler hydrocarbon structure than the other fuels, is less inclined to produce soot. With this knowledge of particle sizes, we were able to calculate the amount of scattering to be expected. Whereas the larger ethylene- and kerosene-generated soot aggregates scatter 20 to 30 percent of the amount of light they absorb, the smaller methane-produced aggregates scatter only 3 percent. This difference accounts for the amount of variation we had found in our measurement of light extinction, implying that the absorption coefficient is the same for the soot from all three types of flames—consistent with our earlier high-resolution TEM study.
Taking the analysis of our laser measurements one step further, we calculated the values of the real and imaginary components of the index of refraction that are necessary to yield the absorption coefficient we had measured, using a relation originally derived by the 19th-century British physicist Lord Rayleigh for the scattering and absorption of light by small particles. In addition, we calculated the index of refraction that would result in the amount of scattering we had measured for ethylene soot. The result was 1.8 - 1.0i, similar to the values recently reported by two other groups, and also similar to the number Janzen arrived at nearly 30 years ago.
With these results, it appears that we are finally arriving at a clear understanding of just how black soot really is: Its characteristic absorptivity is larger, by about a factor of two, than what many researchers had long believed. Soot particles therefore have a greater ability both to absorb and to emit light than was previously realized. The data have brought needed clarity to the understanding of soot optical properties as the particles evolve in both small, steady flames and large, erratic pool fires.
Using these new values for how much light is extinguished as it passes though a cloud of soot, we can better estimate the amount of soot in flames and the temperature of these particles. Knowing the temperature, concentration and emissivity of soot allows one to determine how much radiant heat is transferred from a flame, which in turn may help investigators to understand more fully the dynamics of fires, particularly large accidental ones. With this information, fuel depots might be built to safer standards, and emergency personnel may be able to make better decisions when serious problems arise. But we also hope that such studies can aid in the appreciation of soot, a substance that can be as brilliant and useful as it can be dark and destructive.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.