-
PDF
- Split View
-
Views
-
Cite
Cite
Manabu Morishima, Akira Marui, Shigeki Yanagi, Takamasa Nomura, Naoki Nakajima, Suong-Hyu Hyon, Tadashi Ikeda, Ryuzo Sakata, Sustained release of vancomycin from a new biodegradable glue to prevent methicillin-resistant Staphylococcus aureus graft infection, Interactive CardioVascular and Thoracic Surgery, Volume 11, Issue 1, July 2010, Pages 52–55, https://doi.org/10.1510/icvts.2010.232447
Close - Share Icon Share
Abstract
Prosthetic graft infection with methicillin-resistant Staphylococcus aureus (MRSA) is one of the most serious complications of cardiovascular surgery. Seeking to prevent graft infection, we evaluated the efficacy of a new biodegradable hydrogel glue (new-glue) composed of aldehyded dextran and ε-poly(L-lysine) which acts as a local sustained-release carrier of vancomycin. Rats (n=40) were implanted with 1-cm2 Dacron grafts in the subcutaneous pockets. Groups (n=10 each) were as follows: no treatment (group A), topical vancomycin solution (group B), new-glue without vancomycin (group C) or new-glue containing 1 mg of vancomycin (group D). Twenty-four h after the implantation, 2.0×107 colony-forming units of MRSA was inoculated onto the graft surface. Seven days thereafter, the graft was sampled and cultured. The quantity of MRSA was significantly lower in group D than in the other groups (P<0.0001). About 95% of the total vancomycin was released from the new-glue over the 72 h experimental period, and the tissue concentration of vancomycin remained above the minimum inhibitory concentration for the MRSA strain throughout the experiment. This new vancomycin-containing glue effectively prevented prosthetic graft infection and thus may be a promising biodegradable drug vehicle.
1. Introduction
Prosthetic graft infection is one of the most serious complications of cardiovascular surgery; its incidence is reported to be between 1 and 6% [1, 2]. Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most common and serious causes of prosthetic graft infections [3, 4]. Due to its high mortality rate, preventing MRSA infection is crucial. We have reported on several methods of preventing graft infection using biomaterials [5, 6]. In the present study, we developed a new biodegradable hydrogel glue (new-glue) which can deliver sustained local release of vancomycin. This glue can be produced inexpensively from fungus-derived food additives with no risk of viral infection and low potential for cytotoxicity [7, 8]. The purpose of this study was to evaluate the efficacy of this new-glue for preventing MRSA infection of prosthetic grafts. We evaluated (1) the tissue concentration and release profile of vancomycin and (2) the MRSA count on the infected graft, using a subcutaneous graft-infected rat model [9].
2. Materials and methods
Adult male Wistar rats (body weight: 200–250 g) were purchased from Shimizu Laboratory Supplies Co. Ltd. (Kyoto, Japan). The MRSA strain SR3637 was purchased from Shionogi Co. Ltd. (Osaka, Japan). This study was approved by the animal research committee of Kyoto University and performed in accordance with the European Convention on Animal Care.
2.1. Preparation of new-glue
New-glue is composed of a Schiff base formation of aldehyded dextran and ε-poly(L-lysine) [7]. Briefly, 20 g of dextran (70 KDa, Meiyo Sangyo Co. Ltd., Aichi, Japan) was dissolved in 80 ml of distilled water. Three g of sodium periodate dissolved in 40 ml of water was added to the dextran. The oxidation reaction proceeded at 50 °C for 1 h and was followed by dialysis and air-drying. Then 0.5 g of succinic anhydride was added to 20 g of aqueous ε-poly(L-lysine) (25 w/w%, 4 KDa, Chisso Corp., Tokyo, Japan) and stirred at 50 °C for 1 h to induce acylation. In the process of air-drying and crushing, ε-poly(L-lysine) was recovered with 12 mol% of its amino groups acylated. Ten g of aldehyded dextran (−CHO content=0.30/sugar unit) and 2.5 g of ε-poly(L-lysine) powder were mixed and crushed into a fine powder composed of granules <0.1 mm. This powder could be immediately gelled into new-glue through the addition of water.
In order to turn this new-glue into a vehicle for vancomycin, we shaped it into disks by adding 0.2 ml of physiological saline to 100 mg of new-glue on a silicon mold (diameter: 10 mm; depth: 3 mm). An aqueous solution of vancomycin [1 mg of vancomycin (Shionogi Co. Ltd., Osaka, Japan) dissolved in 0.2 ml of physiological saline] was then added to 100 mg of new-glue on the same silicon mold. All procedures were conducted under sterile conditions.
2.2. Tissue concentration and release profile of vancomycin
Forty-two rats were anesthetized with ether; the hair on their backs was shaved and the skin was sterilized with a 10% povidone-iodine solution. On each side of the median line, one subcutaneous pocket was made with a 1.5-cm incision. The rats were divided into two groups (n=21 each). In the VCM solution group, an aqueous solution of vancomycin (1 mg dissolved in 0.2 ml of physiological saline) was injected into the pockets. In the VCM+glue group, a new-glue disk containing 1 mg of vancomycin was implanted into the subcutaneous pocket; the pocket was then closed with skin clips. At 1, 3, 6, 12, 24, 48, and 72 h after implantation or injection, three rats from each group were euthanized with CO2 and 0.5 g of subcutaneous tissue from the region of the pocket was harvested and stored at –20 °C until measurement. The tissue was homogenized in 2 ml of phosphate-buffered saline for 2 min. The homogenate was centrifuged at 15,000 rpm at 4 °C for 20 min. The vancomycin concentration of the supernatant fluid of the homogenate was measured by fluorescence polarization immunoassay. The limit of detection was <2.0 μg/ml.
In the VCM+glue group, the remaining new-glue disks were also harvested from the subcutaneous pockets at the above-mentioned times. The disks were placed in tubes containing 10 ml of phosphate-buffered saline and incubated at 37 °C for 72 h. As a control, four new-glue disks which contained vancomycin but which had not been implanted in rats were incubated in the same manner. The vancomycin concentration of each sample was measured in a fluorescence polarization immunoassay, and the percentage of vancomycin remaining in the new-glue disk was calculated.
2.3. Evaluation of MRSA count on the infected graft
Forty male rats were anesthetized and given subcutaneous pockets in the manner described above. Under aseptic conditions, 1 cm2 sterile gelatin-impregnated Dacron grafts (Gelweave, Vascutek Ltd., Inchinnan, UK) were implanted into the pockets. The rats were randomly divided into four groups of 10 rats each, according to the type of implant placed in the subcutaneous pockets. The groups received the following treatments: no treatment (group A), topical vancomycin solution (1 mg of vancomycin dissolved in 0.2 ml of physiological saline) (group B), a new-glue disk without medication (group C) or a new-glue disk containing 1 mg of vancomycin (group D). The pockets were closed with skin clips.
Twenty-four h after the implantation of the grafts, the rats were again anesthetized with ether. A sterile phosphate-buffered saline solution (1 ml) containing 2.0×107 colony-forming units (CFU) of the MRSA SR3637 strain was inoculated onto the graft surface by means of a tuberculin syringe to create a subcutaneous fluid-filled pocket [9]. Seven days after MRSA inoculation, rats were euthanized with CO2, and the Dacron grafts were explanted from their subcutaneous tissue. The explanted grafts were washed in sterile saline solution, placed in tubes containing 10 ml of phosphate-buffered saline and sonicated for 2 min to remove the adherent bacteria from the grafts. The bacterial suspensions were diluted serially up to a 10-fold dilution, and 0.1 ml samples were placed on normal agar plates. All plates were incubated at 37 °C for 18 h and evaluated by counting the number of CFU. The limit of detection for this method was <102 CFU/graft.
2.4. Statistical analysis
The experimental results are expressed as means±standard deviations (S.D.). Student's t-test was used to analyze tissue concentrations. Quantitative culture results are presented as log-transformed data. Statistical comparisons between independent groups were performed using analysis of variance on the log-transformed data, and the statistical significance was determined using the Bonferroni/Dunn's method. Statview software (Statview Corp, Cary, NC, USA) was used for all statistical analysis. Statistical significance was defined as P<0.05.
3. Results
3.1. Tissue concentration and release profile of vancomycin
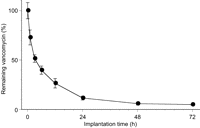
Table 1 shows the results of the portion of the experiment concerned with tissue concentrations of vancomycin. In the VCM+glue group, the concentration of vancomycin was above the minimum inhibitory concentration of the MRSA strain (1.0 μg/ml) for the entire 72 h study period. The tissue concentration of vancomycin in the VCM+glue group was significantly higher than that in the VCM solution group at 6 and 24 h. The percentages of vancomycin remaining in the harvested disks were 72.9±7.6%, 51.5±3.7%, 39.8±4.1%, 26.5±4.7%, 11.6±1.9%, 5.9±1.4%, and 5.0±0.4% at 1, 3, 6, 12, 24, 48, and 72 h, respectively (Fig. 1 ). These results indicate that about 95% of the total vancomycin was released within 72 h of implantation.
Tissue concentrations of vancomycin (μg/ml)
| Time (h) | VCM+glue | VCM solution | P-value |
| group | group | ||
| 1 | 9.2±1.2 | 23.8±10.9 | 0.0824 |
| 3 | 5.9±1.0 | 4.3±2.0 | 0.3093 |
| 6 | 4.9±0.4 | 2.3±0.3 | 0.0046 |
| 12 | 2.7±0.5 | Undetectable | Not available |
| 24 | 2.8±0.1 | 2.2±0.1 | 0.0017 |
| 48 | 3.5±0.3 | Undetectable | Not available |
| 72 | 3.6±0.4 | Undetectable | Not available |
| Time (h) | VCM+glue | VCM solution | P-value |
| group | group | ||
| 1 | 9.2±1.2 | 23.8±10.9 | 0.0824 |
| 3 | 5.9±1.0 | 4.3±2.0 | 0.3093 |
| 6 | 4.9±0.4 | 2.3±0.3 | 0.0046 |
| 12 | 2.7±0.5 | Undetectable | Not available |
| 24 | 2.8±0.1 | 2.2±0.1 | 0.0017 |
| 48 | 3.5±0.3 | Undetectable | Not available |
| 72 | 3.6±0.4 | Undetectable | Not available |
Values are expressed as means±standard deviations (n=3).
VCM+glue, new-glue disk (containing 1 mg of vancomycin) was implanted into the subcutaneous pocket; VCM solution, an aqueous solution of vancomycin (1 mg) was injected into the pocket. The limit of detection was <2.0 μg/ml. VCM, vancomycin.
Tissue concentrations of vancomycin (μg/ml)
| Time (h) | VCM+glue | VCM solution | P-value |
| group | group | ||
| 1 | 9.2±1.2 | 23.8±10.9 | 0.0824 |
| 3 | 5.9±1.0 | 4.3±2.0 | 0.3093 |
| 6 | 4.9±0.4 | 2.3±0.3 | 0.0046 |
| 12 | 2.7±0.5 | Undetectable | Not available |
| 24 | 2.8±0.1 | 2.2±0.1 | 0.0017 |
| 48 | 3.5±0.3 | Undetectable | Not available |
| 72 | 3.6±0.4 | Undetectable | Not available |
| Time (h) | VCM+glue | VCM solution | P-value |
| group | group | ||
| 1 | 9.2±1.2 | 23.8±10.9 | 0.0824 |
| 3 | 5.9±1.0 | 4.3±2.0 | 0.3093 |
| 6 | 4.9±0.4 | 2.3±0.3 | 0.0046 |
| 12 | 2.7±0.5 | Undetectable | Not available |
| 24 | 2.8±0.1 | 2.2±0.1 | 0.0017 |
| 48 | 3.5±0.3 | Undetectable | Not available |
| 72 | 3.6±0.4 | Undetectable | Not available |
Values are expressed as means±standard deviations (n=3).
VCM+glue, new-glue disk (containing 1 mg of vancomycin) was implanted into the subcutaneous pocket; VCM solution, an aqueous solution of vancomycin (1 mg) was injected into the pocket. The limit of detection was <2.0 μg/ml. VCM, vancomycin.
Percentage of vancomycin remaining in the harvested disks. Vancomycin was released from the new-glue disks into the subcutaneous tissue. The x-axis shows the time course, and the y-axis shows the percentage of vancomycin remaining in the harvested disks at 1, 3, 6, 12, 24, 48, 72 h.
3.2. Evaluation of MRSA count on the infected graft
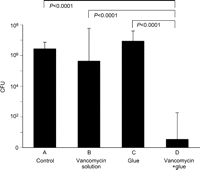
None of the animals died during the study, but all rats in group A and group C showed evidence of graft infection. In group D, nine of the 10 samples were negative for bacterial cultures. In group B, in contrast, only one sample was negative. The log-transformed quantitative culture values for groups A, B, C and D were 6.4±0.4, 5.6±2.1, 6.9±0.7 and 0.55±1.73, respectively. There were significant differences between group D and the other three groups (Fig. 2 ).
MRSA counts on the infected grafts. n=10 (each group). The y-axis shows the numbers of MRSA CFU (logarithmic scale). CFU, colony-forming units; MRSA, methicillin-resistant Staphylococcus aureus.
4. Discussion
This is the first report of the use of new-glue for local sustained drug release. In this study, vancomycin was released from the new-glue over a 72-h period, and the tissue concentrations of vancomycin in the VCM+glue group were significantly higher than those in the VCM solution group. The concentration in the VCM+glue group remained above the minimum inhibitory concentration of MRSA for more than 72 h. The growth of MRSA was drastically inhibited in group D (the VCM+glue group), though there was no inhibitory effect in any of the other three groups.
Our new-glue exhibits a number of favorable characteristics that make it suitable as a drug vehicle [7]. First, it is biocompatible and minimally toxic, and there is no risk of viral infection because it is not made from human plasma or other animal-derived products. The cytotoxicity of aldehyded dextran is as low as 1/1000 of the cytotoxicity of formaldehyde and glutaraldehyde, which are often used as crosslinkers in certain hydrogels and adhesives. In addition, the cytotoxicity of ε-poly(L-lysine) is quite low. Second, this new-glue is biodegradable (it is degraded mainly by hydrolysis). This makes it suitable for use as a drug vehicle, because it does not require a second operation for removal. Third, the degradation rate of the hydrogel can be easily controlled by changing the proportion of the aldehyded group introduced in dextran. Thus, it is easy to prepare different types of glue with longer or shorter periods of drug release. Furthermore, our new-glue is easy to handle: because gel formation occurs soon after water is added, it is easy to add medication and thereby make new-glue into a drug-containing vehicle. It is also easy to modify the dosage in such a vehicle.
In addition, our new-glue has other characteristics which will make it a useful surgical adhesive: it has a high bonding strength and high flexibility (its bonding strength is four times that of fibrin glue). In a previous study, this new-glue demonstrated a strong sealing effect and good biocompatibility when it was applied to pulmonary air leakages in dogs [8].
There have been several animal studies on sustained local release of antibiotics to prevent graft infections. Fibrin glue is often used as a drug carrier for antibiotics, but the release periods it offers are <24 h [10], which is insufficient for this particular therapeutic application. Furthermore, fibrin glue carries a risk of viral infection and is expensive to produce, because its source is human blood. There have also been studies on the use of drug-impregnated grafts, but these as the sole source of antibiotics are often insufficient to prevent graft infection [11, 12]. Rifampicin-soaked gelatin-impregnated prosthetic grafts have recently been used clinically for in-situ replacement of graft infection, but they are not sufficiently effective at preventing MRSA graft infection [13]. Randomized controlled trials have revealed that prophylactic rifampicin-soaked gelatin-impregnated grafts failed to reduce graft infection rates at one month or at two years [2, 14].
In the present study, our new-glue containing vancomycin prevented graft infection with sufficient effectiveness. Nine out of 10 explanted graft samples were negative for bacterial cultures. Achieving the complete eradication of bacteria rather than a simple reduction in their numbers is important to avoid generating a drug-resistant organism.
The current study has some limitations. First, we used rats as our experimental model, and rats might be more resistant to infection than humans are. Second, we used a subcutaneous graft infection model, which does not completely simulate the clinical situation of vascular graft infection. Further studies using the graft-implanted models in larger animals will be required to confirm our conclusions. Third, we used new-glue in the form of disks; it may be more practical to coat graft surfaces with new-glue containing vancomycin.
In conclusion, our new-glue containing vancomycin prevented prosthetic MRSA graft infection. It may be useful in preventive and adjunctive therapy for graft infection. This new-glue appears to have promise as a safe and biodegradable drug vehicle.
This work was financially supported by the Innovation Promotion Program of 2008 from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.