-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Gorlitzer, Florian Wagner, Steffen Pfeiffer, Sandra Folkmann, Johann Meinhart, Theodor Fischlein, Hermann Reichenspurner, Martin Grabenwöger, A prospective randomized multicenter trial shows improvement of sternum related complications in cardiac surgery with the Posthorax® support vest, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 5, May 2010, Pages 714–718, https://doi.org/10.1510/icvts.2009.223305
Close - Share Icon Share
Abstract
Sternal instability, dehiscence and mediastinitis are major causes of morbidity and mortality in cardiac surgery. The aim of this analysis is to determine the effect of a Posthorax® support vest (Epple Inc, Vienna, Austria) after median sternotomy. One thousand five hundred and sixty cases were included in a prospective randomized multicenter trial. Patients were randomized as follows: 905 received a flexible dressing postoperatively (group A) and 655 patients were given a Posthorax® support vest (group B). Patients in groups A and B were well matched. Their mean age was 68 years (range: 34–87 years). The patient characteristics and operative data were equally distributed in both groups. The mean total hospital stay was significantly shorter in group B than in group A (A: 17.33±17.5; B: 14.76±7.7; P=0.04). Sternal wound complications necessitating reoperation during the 90 days follow-up period were observed in 4.5%. Reoperation rates were as follows: 3.9% in group A and 0.6% in group B (P<0.05). The use of the Posthorax® sternum support vest is a valuable adjunct to prevent sternum-related complications after cardiac surgery. In the 90 days follow-up period, additional surgical procedures were significantly reduced by the use of the support vest.
1. Introduction
Instability and infection of median sternotomy wounds are major complications in cardiac surgery due to their association with greater morbidity and mortality, prolonged hospital stays, higher costs, and the need for repeat surgery [1]. Potential perioperative risk factors leading to sternal dehiscence or mediastinitis have been analyzed in several studies; these include diabetes mellitus, chronic obstructive pulmonary disease, obesity, the use of bilateral internal mammary arteries, smoking, and prolonged bypass or ventilation time [1, 2]. The need for repeated blood transfusions in the early postoperative period has also been reported [1]. Advanced age, female gender, the type of cardiac operation, reoperation, steroid therapy, and previous mediastinal irradiation have been identified as important risk factors. Furthermore, the excessive use of bone wax or diathermy may inhibit wound healing [3, 4].
These reports have highlighted the need for careful preventive surgical strategies and perioperative management. The avoidance of a paramedian sternotomy and adequate sternal reinforcement are crucial factors to avoid sternal complications. Additional strategies, such as minimally invasive approaches, the use of a novel sternal synthesis device or biodegradable hemostatic material as an alternative to bone wax should be taken into consideration.
In the present study, we evaluated the effectiveness of a new sternum support vest as an additional tool in postoperative care after median sternotomy to avoid sternum wound related complications.
2. Materials and methods
One thousand five hundred and sixty patients were analyzed in this prospective randomized multicenter trial conducted from September 2007 to August 2009. The work was approved by the Local Ethics Committee. Each patient provided his/her informed consent. Randomization was performed immediately after the operation. The sternum support vest was applied 48 h after the operation, usually after the patient's stay at the intensive care unit (ICU).
Patients were advised to wear the vest for at least six weeks postoperatively. According to the therapy guidelines of the American Society of Hospital Pharmacists Commission on Therapeutics, cefazolin 1 g intravenously was administered every 8 h or every 12 h for 48–72 h or until the chest and mediastinal drainage tubes were removed.
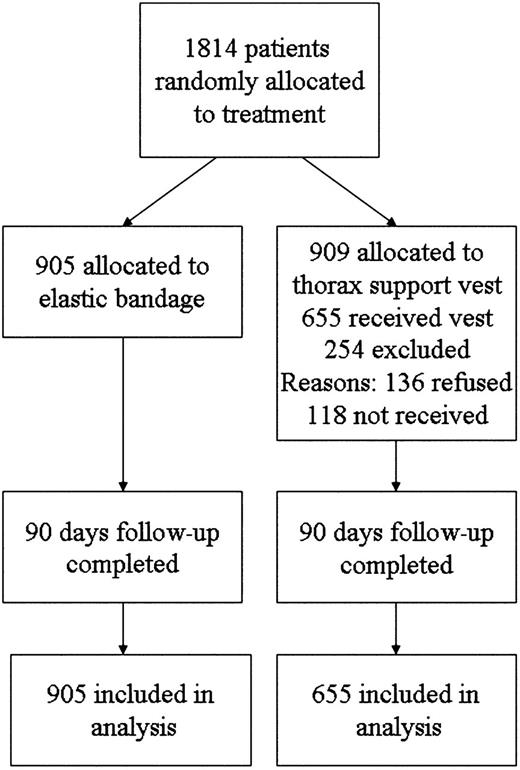
Patients were stratified using a modified Society of Thoracic Surgeons (STS) risk score analysis for major infection after cardiac surgery in order to evaluate their potential infection risk at the surgical site [5]. Exclusion criteria were age under 20 years, the presence of a congenital heart defect, mechanical reanimation and irradiation of the chest. Only patients who actively wore the support vest and fulfilled the inclusion criteria were included in the per-protocol analysis. Patients who were randomized to the vest group and failed or refused to use it were excluded from the study. Reasons for not receiving the vest were prolonged stays at the ICU, an open sternum, or the patient being transferred to a different hospital within 48 h. Slippage and discomfort were the main reasons for declining to use the vest. The flow diagram of all allocated and analyzed patients are summarized in Fig. 1 according to the Consolidated Standards of Reporting Trials (CONSORT) Statement [6].
Patients were monitored for the development of sternal dehiscence or wound infection for 90 days after cardiac surgery. Sternal wound infections included superficial infections involving the skin and subcutaneous tissue of the incision, as well as deep infections. Infection data were obtained during hospitalization from the patients' medical records. During the 90 days follow-up period after discharge, the patients were monitored at their visits to the out-patient clinic or by telephone interviews focusing on sternal wound problems.
2.1. Thorax support vest
The Posthorax® sternum support vest (Epple Inc, Vienna, Austria) is a recently developed, patented design (Fig. 2 ). Its outstanding feature is the fact that pressure and stabilization are specifically aimed towards the sternum. Instead of simple lateral compression, the Posthorax® vest provides anteroposterior stabilization while holding the two halves of the sternum in place. The two cushions are placed longitudinally to the left and the right side of the sternum and serve as shock absorbers when the patient coughs or breathes deeply, and also support the sternum when turning the patient in bed (Fig. 3 ).
Two cushions are placed to the left and the right side of the sternum and serve as shock absorbers.
2.2. Statistical methods
Data were analyzed using the software programs StatXact, Version 8 (Cytel Inc) and Statistica, Version 8 (StatSoft Inc, Tulsa, OK).
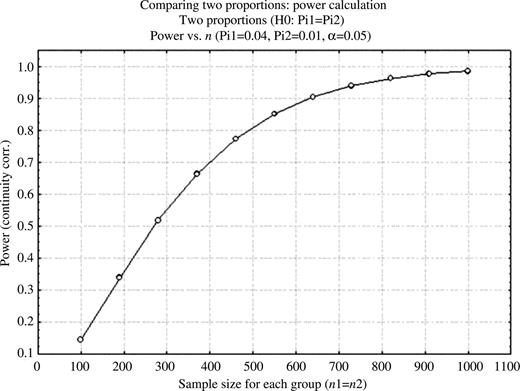
Based on a clinically relevant difference of cumulative complication rates of 4% in group A and 1% in group B the projected sample size at a power of 0.8–0.9 is ∼650 per group (Fig. 4 ). Statistically significant differences were calculated according to the following scales:
Nominal scale* – descriptive statistics: total sum, percent; Test for comparisons between two groups: Pearson's χ2-test.
Ordinal scale** – descriptive statistics: median, interquartile range (IQR); Calculation with Mann–Whitney U-test.
Interval scale*** – descriptive statistics: mean, standard deviation (S.D.); Test for comparisons between groups: Mann–Whitney U-test.
P<0.05 were considered to indicate significance.
3. Results
From September 2007 onward, patients who fulfilled the inclusion criteria were randomized into two groups: group A did not receive the thorax support vest and was treated with an elastic bandage (n=905) while group B received the vest for stabilization of the sternum (n=655). Patients, who refused the vest or did not receive it within 48 h for other reasons, were excluded from the study.
Clinical features of the patients are shown in Table 1 . No significant difference was registered between the two randomized groups A and B with regard to age, gender, body mass index, EuroSCORE and potential risk factors, such as diabetes, chronic renal failure, chronic obstructive pulmonary disease, peripheral artery disease, cardiogenic shock, myocardial infarction, organic brain syndrome, congestive heart failure and the use of red cell units. The modified STS infection risk score [5] and the New York Heart Association classification were equally distributed in both groups.
Patient characteristics
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Age*** | 67.49 (±10.9) | 66.83 (±10.7) | 0.76 |
| Size*** | 170 (±12.6) | 173.85 (±64.2) | 0.82 |
| Weight*** | 81.96 (±16.7) | 81.27 (±15) | 0.82 |
| BMI*** | 30.38 (±29.7) | 27.58 (±4.5) | 1.00 |
| Red cell units*** | 1.05 (±1.9) | 0.95 (±1.8) | 1.00 |
| Male* | 69.9% | 71.6% | 0.54 |
| Female* | 29.9% | 28.4% | 0.54 |
| Diabetes* | 28.7% | 30.5% | 0.46 |
| CRF* | 10.1% | 8.2% | 0.25 |
| COPD* | 17.3% | 17.3% | 1.00 |
| PAD* | 8.8% | 9.5% | 0.72 |
| Cardiogenic shock* | 1.2% | 1.1% | 1.00 |
| MCI* | 20.0% | 22.1% | 0.31 |
| OBS* | 11.9% | 10.5% | 0.42 |
| CHF* | 4.20% | 5.04% | 0.46 |
| Infection risk score** | 9.00 (±7) | 8.00 (±7) | 1.00 |
| NYHA** | 3.00 (±1) | 3.00 (±1) | 0.25 |
| EuroSCORE linear** | 5.00 (±2.9) | 4.00 (±2.7) | 0.91 |
| EuroSCORE logistic** | 3.99 (±6.5) | 3.59 (±4.9) | 0.63 |
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Age*** | 67.49 (±10.9) | 66.83 (±10.7) | 0.76 |
| Size*** | 170 (±12.6) | 173.85 (±64.2) | 0.82 |
| Weight*** | 81.96 (±16.7) | 81.27 (±15) | 0.82 |
| BMI*** | 30.38 (±29.7) | 27.58 (±4.5) | 1.00 |
| Red cell units*** | 1.05 (±1.9) | 0.95 (±1.8) | 1.00 |
| Male* | 69.9% | 71.6% | 0.54 |
| Female* | 29.9% | 28.4% | 0.54 |
| Diabetes* | 28.7% | 30.5% | 0.46 |
| CRF* | 10.1% | 8.2% | 0.25 |
| COPD* | 17.3% | 17.3% | 1.00 |
| PAD* | 8.8% | 9.5% | 0.72 |
| Cardiogenic shock* | 1.2% | 1.1% | 1.00 |
| MCI* | 20.0% | 22.1% | 0.31 |
| OBS* | 11.9% | 10.5% | 0.42 |
| CHF* | 4.20% | 5.04% | 0.46 |
| Infection risk score** | 9.00 (±7) | 8.00 (±7) | 1.00 |
| NYHA** | 3.00 (±1) | 3.00 (±1) | 0.25 |
| EuroSCORE linear** | 5.00 (±2.9) | 4.00 (±2.7) | 0.91 |
| EuroSCORE logistic** | 3.99 (±6.5) | 3.59 (±4.9) | 0.63 |
*Nominal scale (in percentage): using Pearson's χ2-test.
**Ordinal scale (median±IQR): using Mann–Whitney U-test.
***Interval scale (mean±S.D.): using Mann–Whitney U-test.
BMI, body mass index; CRF, chronic renal failure; PAD, peripheral artery disease; MCI, myocardial infarction; OBS, organic brain syndrome; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association classification; IQR, interquartile range; S.D., standard deviation.
Patient characteristics
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Age*** | 67.49 (±10.9) | 66.83 (±10.7) | 0.76 |
| Size*** | 170 (±12.6) | 173.85 (±64.2) | 0.82 |
| Weight*** | 81.96 (±16.7) | 81.27 (±15) | 0.82 |
| BMI*** | 30.38 (±29.7) | 27.58 (±4.5) | 1.00 |
| Red cell units*** | 1.05 (±1.9) | 0.95 (±1.8) | 1.00 |
| Male* | 69.9% | 71.6% | 0.54 |
| Female* | 29.9% | 28.4% | 0.54 |
| Diabetes* | 28.7% | 30.5% | 0.46 |
| CRF* | 10.1% | 8.2% | 0.25 |
| COPD* | 17.3% | 17.3% | 1.00 |
| PAD* | 8.8% | 9.5% | 0.72 |
| Cardiogenic shock* | 1.2% | 1.1% | 1.00 |
| MCI* | 20.0% | 22.1% | 0.31 |
| OBS* | 11.9% | 10.5% | 0.42 |
| CHF* | 4.20% | 5.04% | 0.46 |
| Infection risk score** | 9.00 (±7) | 8.00 (±7) | 1.00 |
| NYHA** | 3.00 (±1) | 3.00 (±1) | 0.25 |
| EuroSCORE linear** | 5.00 (±2.9) | 4.00 (±2.7) | 0.91 |
| EuroSCORE logistic** | 3.99 (±6.5) | 3.59 (±4.9) | 0.63 |
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Age*** | 67.49 (±10.9) | 66.83 (±10.7) | 0.76 |
| Size*** | 170 (±12.6) | 173.85 (±64.2) | 0.82 |
| Weight*** | 81.96 (±16.7) | 81.27 (±15) | 0.82 |
| BMI*** | 30.38 (±29.7) | 27.58 (±4.5) | 1.00 |
| Red cell units*** | 1.05 (±1.9) | 0.95 (±1.8) | 1.00 |
| Male* | 69.9% | 71.6% | 0.54 |
| Female* | 29.9% | 28.4% | 0.54 |
| Diabetes* | 28.7% | 30.5% | 0.46 |
| CRF* | 10.1% | 8.2% | 0.25 |
| COPD* | 17.3% | 17.3% | 1.00 |
| PAD* | 8.8% | 9.5% | 0.72 |
| Cardiogenic shock* | 1.2% | 1.1% | 1.00 |
| MCI* | 20.0% | 22.1% | 0.31 |
| OBS* | 11.9% | 10.5% | 0.42 |
| CHF* | 4.20% | 5.04% | 0.46 |
| Infection risk score** | 9.00 (±7) | 8.00 (±7) | 1.00 |
| NYHA** | 3.00 (±1) | 3.00 (±1) | 0.25 |
| EuroSCORE linear** | 5.00 (±2.9) | 4.00 (±2.7) | 0.91 |
| EuroSCORE logistic** | 3.99 (±6.5) | 3.59 (±4.9) | 0.63 |
*Nominal scale (in percentage): using Pearson's χ2-test.
**Ordinal scale (median±IQR): using Mann–Whitney U-test.
***Interval scale (mean±S.D.): using Mann–Whitney U-test.
BMI, body mass index; CRF, chronic renal failure; PAD, peripheral artery disease; MCI, myocardial infarction; OBS, organic brain syndrome; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association classification; IQR, interquartile range; S.D., standard deviation.
The surgical data (summarized in Table 2 ) revealed no difference between groups A and B, except for the rate of aortic valve replacement, which was higher in group A (A: 18.2% vs. B: 16.3%, P=0.03).
Operative data
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Ventilation hours** | 23.61 (±78.3) | 11.35 (±9.11) | 0.13 |
| ICU** | 3.05 (±3.8) | 2.55 (±3.0) | 0.12 |
| LOS** | 17.33 (±17.5) | 14.76 (±7.7) | 0.04 |
| Concomitant surgery* | 27.3% | 28.2% | 0.69 |
| p100* | 39.8% | 41.4% | 0.53 |
| p200* | 4.4% | 3.4% | 0.36 |
| CABG* | 52.2% | 56.0% | 0.22 |
| CABG bimammaria* | 2.5% | 2.6% | 1 |
| AKE* | 18.2% | 16.3% | 0.03 |
| MKE/R* | 8.6% | 6.6% | 0.08 |
| AKE+CABG* | 12.2% | 11.6% | 0.58 |
| MKE/R+CABG* | 2.5% | 4.0% | 0.18 |
| AKE+MKE/R* | 1.7% | 0.5% | 0.05 |
| Aneurysm* | 2.1% | 2.4% | 0.86 |
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Ventilation hours** | 23.61 (±78.3) | 11.35 (±9.11) | 0.13 |
| ICU** | 3.05 (±3.8) | 2.55 (±3.0) | 0.12 |
| LOS** | 17.33 (±17.5) | 14.76 (±7.7) | 0.04 |
| Concomitant surgery* | 27.3% | 28.2% | 0.69 |
| p100* | 39.8% | 41.4% | 0.53 |
| p200* | 4.4% | 3.4% | 0.36 |
| CABG* | 52.2% | 56.0% | 0.22 |
| CABG bimammaria* | 2.5% | 2.6% | 1 |
| AKE* | 18.2% | 16.3% | 0.03 |
| MKE/R* | 8.6% | 6.6% | 0.08 |
| AKE+CABG* | 12.2% | 11.6% | 0.58 |
| MKE/R+CABG* | 2.5% | 4.0% | 0.18 |
| AKE+MKE/R* | 1.7% | 0.5% | 0.05 |
| Aneurysm* | 2.1% | 2.4% | 0.86 |
*Nominal scale (in percentage): using Pearson's χ2-test.
**Interval scale (mean±S.D.): using Mann–Whitney U-test.
ICU, intensive care unit stay (days); LOS, length of stay in hospital (days); p100, perfusion time 100–200 min; p200, perfusion time 200–300 min; CABG, coronary artery bypass graft; CABG bimammaria, CABG using both mammary arteries; AKE, aortic valve replacement; MKE/R, mitral valve reconstruction/replacement; S.D., standard deviation.
Operative data
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Ventilation hours** | 23.61 (±78.3) | 11.35 (±9.11) | 0.13 |
| ICU** | 3.05 (±3.8) | 2.55 (±3.0) | 0.12 |
| LOS** | 17.33 (±17.5) | 14.76 (±7.7) | 0.04 |
| Concomitant surgery* | 27.3% | 28.2% | 0.69 |
| p100* | 39.8% | 41.4% | 0.53 |
| p200* | 4.4% | 3.4% | 0.36 |
| CABG* | 52.2% | 56.0% | 0.22 |
| CABG bimammaria* | 2.5% | 2.6% | 1 |
| AKE* | 18.2% | 16.3% | 0.03 |
| MKE/R* | 8.6% | 6.6% | 0.08 |
| AKE+CABG* | 12.2% | 11.6% | 0.58 |
| MKE/R+CABG* | 2.5% | 4.0% | 0.18 |
| AKE+MKE/R* | 1.7% | 0.5% | 0.05 |
| Aneurysm* | 2.1% | 2.4% | 0.86 |
| Non-vest | Vest group | P-value | |
| (n=905) | (n=655) | ||
| Ventilation hours** | 23.61 (±78.3) | 11.35 (±9.11) | 0.13 |
| ICU** | 3.05 (±3.8) | 2.55 (±3.0) | 0.12 |
| LOS** | 17.33 (±17.5) | 14.76 (±7.7) | 0.04 |
| Concomitant surgery* | 27.3% | 28.2% | 0.69 |
| p100* | 39.8% | 41.4% | 0.53 |
| p200* | 4.4% | 3.4% | 0.36 |
| CABG* | 52.2% | 56.0% | 0.22 |
| CABG bimammaria* | 2.5% | 2.6% | 1 |
| AKE* | 18.2% | 16.3% | 0.03 |
| MKE/R* | 8.6% | 6.6% | 0.08 |
| AKE+CABG* | 12.2% | 11.6% | 0.58 |
| MKE/R+CABG* | 2.5% | 4.0% | 0.18 |
| AKE+MKE/R* | 1.7% | 0.5% | 0.05 |
| Aneurysm* | 2.1% | 2.4% | 0.86 |
*Nominal scale (in percentage): using Pearson's χ2-test.
**Interval scale (mean±S.D.): using Mann–Whitney U-test.
ICU, intensive care unit stay (days); LOS, length of stay in hospital (days); p100, perfusion time 100–200 min; p200, perfusion time 200–300 min; CABG, coronary artery bypass graft; CABG bimammaria, CABG using both mammary arteries; AKE, aortic valve replacement; MKE/R, mitral valve reconstruction/replacement; S.D., standard deviation.
Complications were divided into dehiscence, superficial wound complication, and deep sternal infection. All complications required reoperation, including implantation of VAC® systems (KCI Inc, San Antonio, Texas), surgical debridement, re-cerclage, or closure of the chest with a pectoralis muscle flap. Complications occurring during the hospital stay, readmissions and reoperation rates within 90 days were evaluated according to the study protocol. The rate of sternal site infections and dehiscence are summarized in Table 3 .
Rate of complications
| Non-vest (%) | Vest group (%) | P-value | Power | |
| (n=905) | (n=655) | |||
| Complication rate during hospital stay | ||||
| Dehiscence | 0.55 | 0.00 | 0.08 | 0.272 |
| Superficial wound infection | 0.88 | 0.46 | 0.38 | 0.088 |
| Deep sternal infection | 1.10 | 0.00 | 0.007 | 0.624 |
| Cummulative | 2.54 | 0.46 | 0.002 | 0.870 |
| Complication rate 90 days follow-up | ||||
| Dehiscence | 0.22 | 0.00 | 0.51 | 0.973 |
| Superficial wound infection | 0.22 | 0.15 | 1 | 0.012 |
| Deep sternal infection | 0.88 | 0.00 | 0.02 | 0.540 |
| Cummulative | 1.33 | 0.15 | 0.02 | 0.628 |
| Total complication rate | ||||
| Dehiscence | 0.77 | 0.00 | 0.046 | 0.456 |
| Superficial wound infection | 1.10 | 0.61 | 0.418 | 0.101 |
| Deep sternal infection | 1.99 | 0.00 | 0.0001 | 0.955 |
| Cummulative | 3.87 | 0.61 | 0.047 | 0.980 |
| Non-vest (%) | Vest group (%) | P-value | Power | |
| (n=905) | (n=655) | |||
| Complication rate during hospital stay | ||||
| Dehiscence | 0.55 | 0.00 | 0.08 | 0.272 |
| Superficial wound infection | 0.88 | 0.46 | 0.38 | 0.088 |
| Deep sternal infection | 1.10 | 0.00 | 0.007 | 0.624 |
| Cummulative | 2.54 | 0.46 | 0.002 | 0.870 |
| Complication rate 90 days follow-up | ||||
| Dehiscence | 0.22 | 0.00 | 0.51 | 0.973 |
| Superficial wound infection | 0.22 | 0.15 | 1 | 0.012 |
| Deep sternal infection | 0.88 | 0.00 | 0.02 | 0.540 |
| Cummulative | 1.33 | 0.15 | 0.02 | 0.628 |
| Total complication rate | ||||
| Dehiscence | 0.77 | 0.00 | 0.046 | 0.456 |
| Superficial wound infection | 1.10 | 0.61 | 0.418 | 0.101 |
| Deep sternal infection | 1.99 | 0.00 | 0.0001 | 0.955 |
| Cummulative | 3.87 | 0.61 | 0.047 | 0.980 |
Rate of complications
| Non-vest (%) | Vest group (%) | P-value | Power | |
| (n=905) | (n=655) | |||
| Complication rate during hospital stay | ||||
| Dehiscence | 0.55 | 0.00 | 0.08 | 0.272 |
| Superficial wound infection | 0.88 | 0.46 | 0.38 | 0.088 |
| Deep sternal infection | 1.10 | 0.00 | 0.007 | 0.624 |
| Cummulative | 2.54 | 0.46 | 0.002 | 0.870 |
| Complication rate 90 days follow-up | ||||
| Dehiscence | 0.22 | 0.00 | 0.51 | 0.973 |
| Superficial wound infection | 0.22 | 0.15 | 1 | 0.012 |
| Deep sternal infection | 0.88 | 0.00 | 0.02 | 0.540 |
| Cummulative | 1.33 | 0.15 | 0.02 | 0.628 |
| Total complication rate | ||||
| Dehiscence | 0.77 | 0.00 | 0.046 | 0.456 |
| Superficial wound infection | 1.10 | 0.61 | 0.418 | 0.101 |
| Deep sternal infection | 1.99 | 0.00 | 0.0001 | 0.955 |
| Cummulative | 3.87 | 0.61 | 0.047 | 0.980 |
| Non-vest (%) | Vest group (%) | P-value | Power | |
| (n=905) | (n=655) | |||
| Complication rate during hospital stay | ||||
| Dehiscence | 0.55 | 0.00 | 0.08 | 0.272 |
| Superficial wound infection | 0.88 | 0.46 | 0.38 | 0.088 |
| Deep sternal infection | 1.10 | 0.00 | 0.007 | 0.624 |
| Cummulative | 2.54 | 0.46 | 0.002 | 0.870 |
| Complication rate 90 days follow-up | ||||
| Dehiscence | 0.22 | 0.00 | 0.51 | 0.973 |
| Superficial wound infection | 0.22 | 0.15 | 1 | 0.012 |
| Deep sternal infection | 0.88 | 0.00 | 0.02 | 0.540 |
| Cummulative | 1.33 | 0.15 | 0.02 | 0.628 |
| Total complication rate | ||||
| Dehiscence | 0.77 | 0.00 | 0.046 | 0.456 |
| Superficial wound infection | 1.10 | 0.61 | 0.418 | 0.101 |
| Deep sternal infection | 1.99 | 0.00 | 0.0001 | 0.955 |
| Cummulative | 3.87 | 0.61 | 0.047 | 0.980 |
The cumulative sternal wound complication rate was 3.0% during the hospital stay and 1.5% during the 90 days follow-up period. Dehiscence occurred in 0.77% (in-hospital: 0.55%, 90 days: 0.22%), superficial wound complications in 1.7% (in-hospital: 1.3%, 90 days: 0.4%), and deep sternal infections in 2% (in-hospital: 1.1%, 90 days: 0.9%) covering 1560 patients.
Comparing the two randomized groups [non-vest (A) and vest (B)], the difference in the numbers of total complications including the 90 days follow-up period are significant (A: 3.87% vs. B: 0.61%, P=0.047, power=0.98). Patients treated with the thorax support vest had a significantly lower incidence of dehiscence (A: 0.77% vs. B: 0%, P=0.046; power=0.46) and deep sternal infections (A: 1.99% vs. B: 0%, P=0.0001; power=0.95). The occurrence of superficial wound infections revealed no significant difference (A: 1.1% vs. B: 0.6%, P=0.4178; power=0.1).
Hospitalization time including complications was significantly shorter in the vest group (14.76±7.7) than in the non-vest group (17.33±17.5, P=0.04). No significant difference was registered regarding ICU stay between the patient groups (A: 3.05±3.8; B: 2.55±3; P=0.12).
Out of all patients with sternal wound complications 13 (33%) developed their wound infection or dehiscence within 90 days after being discharged from the hospital. The mean duration of the hospital stay was extended to 59.6 days when a patient required repeat surgery due to instability or infection. In cases of readmission during the follow-up period, the mean hospital stay was 41.2 days.
4. Discussion
Sternal wound complications after cardiac operations are associated with higher morbidity, mortality, and several other disadvantages for the patient and the clinician.
The aim of this prospective randomized multicenter trial was to evaluate the effectiveness of a sternum support vest to prevent sternal wound complications in patients undergoing cardiac surgery via median sternotomy. The total rate of 2.9% regarding dehiscence or wound infection during hospital stay registered in the present study is comparable to the data reported in other investigations [7]. The main finding of this study is the reduction of deep sternal infections and dehiscence by using the sternum support vest. No difference can be found preventing reoperation because of postoperative superficial wound infections. There is a wide variation in incidence of superficial sternal wound complications between studies and ranges between 0.9% and 20%. This variation can be due to different classification, follow-up period or surgical management. In the present study, we focused on the wound complications requiring consecutive surgical revisions. The rate of operative reintervention in superficial wound infections is reported between 18% and 30% in these cases, whereas all patients with deep sternal infections underwent one or more reoperation [8–10]. The results of this study underline the importance of mechanical stabilization to reduce the forces that cause movement of the sternum halves or dehiscence. These data support the effectiveness of the sternum support vest to prevent instability and deep sternal infection.
The increase of wound complications after hospital discharge is remarkable. The rate of post-discharge sternal wound infection during a 90-day observation period has been reported to be as high as 79% by other authors [10]. In the present series, 33% of all patients who developed sternum related complications, were registered after hospital discharge. Late onset of sternal wound infection has a poorer prognosis than early onset [11]. This underlines the importance of post-discharge surveillance, especially in patients with multiple morbidities. The long period of time during which postoperative complications may occur is indicative of the fact that postoperative wound management is more important than surgery-related complications or risk factors [10, 12]. Our experience shows that qualified care by a nurse working for 30 h per week/1000 cardiac procedures per year is a prerequisite for effective postoperative care. The economic benefits of shorter hospital stays and lower rates of reoperation would exceed, or at least balance, the additional cost of vest and personnel.
Sternal surgical site infections are one of the most expensive complications in cardiac surgery. In addition to longer hospital stay, indirect costs, such as the need for more antibiotics, wound dressing, and subsequent surgery must be taken into account. Speir and co-authors [13] reported 240% higher costs due to mediastinitis after isolated coronary artery bypass grafting, which is comparable to the data reported from the US Medicare Program, where the increase in the utilization of hospital resources in cases of postoperative infection amounted to 227% [14].
The impact of costs in European hospitals is difficult to assess due to a variety of reimbursement systems used. According to an assessment performed in Helsinki (Finland), the financial burden of postoperative infection was €6,200 [15].
In the present study, the mean duration of the hospital stay in patients with infection at the surgical site after open heart surgery was 44±9 days.
In conclusion, this prospective randomized trial shows that the sternum support vest is a useful aid in postoperative care after median sternotomy. It was found to reduce deep sternal wound complications and, therefore, also a reduction of hospitalization time. Comparative rates of sternal wound problems and subsequent surgery in various risk patient groups will be investigated in future studies.
Presented at the 23rd Annual Meeting of the European Association for Cardio-thoracic Surgery, Vienna, Austria, October 18–21, 2009.
References
Conference discussion
Dr. M. Kolowca (Rzeszow, Poland): I am following with enthusiasm your research on the chest vest. Last year you presented the preliminary results and then you published them in the European Journal and now you presented the results of a much bigger trial. The power of this trial is its design – this is a prospective multicenter randomized trial with 2000 patients enrolled in this study. I have got three comments concerning this research.
The first one is regarding the title of your research. You said in the title that this trial showed improvement of mechanical stress, but you did not study this issue in your research, it is not coming from your research; you didn't touch this problem. What you have in your results are just clinical results, complications, rate of complications and the hospital length of stay, but there was nothing about mechanical stress. So maybe the better place for it is in the discussion where you could say that the clinical results could be connected with the improvement of mechanical stress.
Second one. When you are looking into the results, I have got here Table 3 from the manuscript I received from you. In the vest group where you are using this vest there is no dehiscence, 0.0 during the hospital stay and after 90 days in the group of 655 patients. Very good results. Deep sternal infection also 0.0. On the other hand, in the control group we have got quite a low rate of dehiscence. So it doesn't reach a significant difference in the dehiscence during the hospital stay and after 90 days. The same in the superficial wound infection; there is no difference. The only one difference you show is in deep sternal infection. And this makes the cumulative difference between all the complications only the deep sternal wound infection rate. And in your results it is 1.99, nearly 2%, which is a little bit higher than in the literature. I looked in the literature, and Tirone David reported 0.7%, Professor Carrel from Switzerland 0.9. So it is nearly twice the results reported in the papers. So maybe this is biased by the elastic bandage that people in Group I were wearing. So maybe these results are a little bit biased. I don't know. This is my question.
And the third one is maybe you should consider a six-month follow-up, because three months is just the borderline where we are waiting for healing of the sternum.
Dr. Gorlitzer: To your first comment regarding the title, we performed biomechanical tests with pressure points at the thorax wall, which shows a major difference between the elastic bandage and the support vest.
The second question is focussing the difference in superficial wound infections and dehiscence. This vest actually does not prevent superficial wound healing but prevents sternum infection. Furthermore, we find prevention of dehiscence during 90 days follow-up.
We are planning six-month follow-up because we see a lot of patients have problems after 90 days, even after four or five months.