Abstract
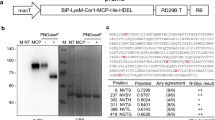
We adapted a previously described Agrobacterium-mediated transient expression system to test the expression level of three constructs carrying the surface antigen 1 (SAG1) of Toxoplasma gondii. Two constructs were based in a Potato virus X (PVX) amplicon. In one of them, the PVX movement protein genes were replaced by the sag1 gene. In the other, the sag1 gene was placed under the control of an additional coat protein subgenomic promoter. In the third construct, the sag1 gene was fused to an apoplastic peptide signal under the CaMV 35S promoter. Western blot analysis of leaf extracts infiltrated with each construct revealed a protein of 35 kDa. SAG1 accumulation in leaves ranged from 0.1 to 0.06% of total soluble protein (equivalent to 10 µg and 6 µg of SAG1 per gram of fresh leaf tissue, respectively). Three of five human seropositive samples reacted with tobacco-expressed SAG1 in Western blot analysis. The C3H mice were immunized with SAG-expressing leaf extracts and perorally challenged with a nonlethal dose of the T. gondii Me49 strain. Mice vaccinated with SAG1 showed significantly lower brain cyst burdens compared to those from the control group. Immunization with SAG1-expressing leaves elicited a specific humoral response with predominant participation of type IgG2a. In conclusion, a functional SAG1 version could be transiently expressed in tobacco leaves.
Similar content being viewed by others
References
Mason, H. S., Lam, D. M., and Arntzen, C. J. (1992) Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 89, 11745–11749.
Giddings, G., Allison, G., Brooks, D., and Carter, A. (2000) Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 18, 1151–1155.
Daniell, H., Streatfield, S. J., and Wycoff, K. (2001) Medical molecular farming: production of antibodies, biopharmaceutical and edible vaccines in plants. Trends Plant Sci. 6, 219–226.
Streatfield, S. J. and Howard, J. A. (2003) Plant-based vaccines. Int. J. Parasitol. 33, 479–493.
Arakawa, T., Yu, J., Chong, D. K., Hough, J., Engel, P. C., and Langridge, W. H. (1998) A plant-based cholera toxin B subunnit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 16, 934–938.
Mason, H. S., Haq, T. A., Clements, J. D., and Arntzen, C. J. (1998) Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a systhetic LT-B gene. Vaccine 16, 1336–1343.
Richter, L. J., Thanavala, Y., Arntzen, C. J., and Mason, H. S. (2000) Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 18, 1167–1171.
Lauterslager, T. G. M., Florack, D. E. A., van der Wal, T. J., Molthoff, J. W., Langeveld, J. P. M., Bosch, D., et al. (2001) Oral immunization of naive and primed animals with transgenic potato tubers expressing LT-B. Vaccine 19, 2749–2755.
Huang, Y., Liang, W., Pan, A., Zhou, Z., Huang, C., Chen, J., et al. (2003) Production of FaeG, the mejor subunit of K88 fimbriae, in transgenic tobacco plants and its immunogenicity in mice. Infect. Immun. 71, 5436–5439.
Perź-Filgueira, D. M., Zamorano, P. I., Domńguez, M. G., Taboga, O., Del Mf—dico Zajac, M. P., Puntel, M., et al. (2003) Bovine herpes virus gD protein produced in plants using a recombinnant tobacco mosaic virus (TMV) vector possesses authentic antigenicity. Vaccine 21, 4201–4209.
Fischer, R., Vaquero-Martin, C., Sack, M., Drossard, J., Emans, N., and Commandeur, U. (1999) Towards molecular farming in the future: transient protein expression in plants. Biotechnol. Appl. Biochem. 30, 113–116.
Sala, F., Rigano, M. M., Barbante, A., Basso, B., Walmsley, A. M., and Castiglione, S. (2003) Vaccine antigen production in transgenic plants: strategies, gene constructs and perspective. Vaccine 21, 803–808.
Kapila, J., De Rycke, R., van Montagu, M., and Angenon, G. (1997) An Agrobacterium-mediated transient gene expression system in intact leaves. Plant Sci. 122, 101–108.
Tenter, A. M., Heckeroth, A. R., and Weiss, L. M. (2000) Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258.
Dubey, J. P. (1990) Status of toxoplasmosis in sheep and goats in the United States. J. Am. Vet. Med. Assoc. 196, 259–262.
Duncanson, P., Terry, R. S., Smith, J. E., and Hide, G. (2001) High levels of congenital transmission of Toxoplasma gondii in a commercial sheep flock. Int. J. Parasitol. 31, 1699–1703.
Frenkel, J. K. and Escajadillo, A. (1987) Cyst rupture as a pathogenic mechanism of toxoplasmic encephalitis. Am. J. Trop. Med. Hyg. 36, 517–522.
Bout, D. T., Méěec, M. N. Velge-Roussel, F., Dimier-Poisson, I., and Lebrun, M. (2002) Prospects for a human Toxoplasma vaccine. Curr. Drug. Targets Immune Endocr. Metabol. Disord. 2, 227–234.
Bhopale, G. M. (2003) Development of a vaccine for toxoplasmosis: current status. Microb. Infect. 5, 457–462.
Nagel, S. D. and Boothroyd, J. C. (1989) The mayor surface antigen, P30, of Toxoplasma gondii anchored by a glycolipid. J. Biol. Chem. 264, 5569–5574.
Skryabin, K. G., Kraev, A. S., Morosov, S. Y., Rosanov, M. N., Chernov, B. K., Lukasheva, L. I., et al. (1988) The nucleotide sequence of potato virus X RNA. Nucleic Acids Res. 16, 10929–10930.
Chapman, S., Hills, G., Watts, J., and Baulcombe, D. (1992) Virus X as a vector for gene expression in plants. Plant J. 2, 549–557.
Santa Cruz, S., Chapman, S., Roberts, A. G., Roberts, I. R., Prior, D. A. M., and Oparka, K. J. (1996) Assembly and movement of a plant virus carrying a green fluorescent protein overcoat. Proc. Natl. Acad. Sci. USA 93, 6286–6290.
Franconi, R., Di Bonito, P., Dibello, F., Accardi, L., Muller, A., Cirilli, A., et al. (2002) Plant-derived human papillomavirus 16 E7 oncoprotein induces immune response and specific tumor protection. Cancer Res. 62, 3654–3658.
Orman, B. E., Celnik, R. M., Mandel, M. A., Torres, T. N., and Mentaberry, A. N. (1991) Complete nucleotide sequence of a South American isolate of potato virus X. Virus Res. 16, 293–305.
Nigro, M., Gutierrez, A., Hoffer, A. M., Clemente, M., Kanfer, F., Carral, L., et al. (2003) Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn. Microbiol. Infect. Dis. 47, 609–613.
Mallory, A. C., Parks, G., Endres, M. W., Baulcombe, D., Bowman, L. H., Pruss, G. J., and Vance, V. B. (2002) The amplicon-plus system for high-level expression of transgenes in plants. Nat. Biothecnol. 20, 622–625.
Brigneti, G., Voinnet, O., Li, W. X., Ji, L., Ding, S. W., and Baulcombe, D. (1998) Viral pathogenicity determinant are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 17, 6739–6746.
Harning, D., Spenter, J., Metsis, A., Vuust, J., and Petersen, E. (1996) Recombinant Toxoplasma gondii surface Antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin. Diagn. Lab. Immunol. 3, 355–357.
Chen, X. G., Gong, Y. H. U., Lun, Z. R., and Fung, M. C. (2001) High- level expression and purification of immunogenic recombinant SAG1 (p30) of Toxoplasma gondii in Escherichia coli. Protein Expr. Purif. 23, 33–37.
Letourner, O., Gervasi, G., Gaia, S., Pages, J., Watelet, B., and Jolivet, M. (2001) Characterization of Toxoplasma gondii surface antigen 1 (SAG1) secreted from Pichia pastoris: evidence of hyper O-glycosylation. Biotechnol. Appl. Biochem. 33, 35–45.
Marti, M., Li, Y., Kohler, P., and Hehl, A. B. (2002) Conformationally correct expression of membraneanchored Toxoplasma gondii SAG1 in the primitive protozoan Giardia duodenalis. Infect. Immunol. 70, 1014–1016.
Kim, K., Bulow, R., Kampmeier, J., and Boothroyd, J. C. (1994) Conformationally appropriate expression of the Toxoplasma antigen SAG1 (p30) in CHO cells. Infect. Immunol. 62, 203–209.
Meek, B., Diepersloot, R. J., Van Gool, T., Speijer, D., and Peek, R. (2003) IgM recognition of recombinant Toxoplasma gondii antigens by sera of acutely or latently infected humans. Diagn. Microbiol. Infect. Dis. 45, 45–52.
Biemans, R., Gregoire, D., Haumont, M., Bosseloir, A., Garcia, L., Jacquet, L., et al. (1998) The conformation of purified Toxoplasma gondii SAG1 antigen, secreted from engineered Pichia pastoris, is adequate for serorecognition and cell proliferation. J. Biotechnol. 66, 137–146.
Vaquero, C., Sack, M., Chandler, J., Drossard, J., Schuster, F., Moneck, M., et al. (1999) A carcinoembryonic antigen-specific antibody produced in tobacco. Proc. Natl. Acad. Sci. USA 96, 11128–11133.
Desolme, B., Méěec, M. N., Buzoni-Gatel, D., and Bout, D. (2000) Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii Gra4 gene. Vaccine 18, 2512–2521.
Vercammen, M., Scorza, T., Huygen, K., De Braekeleer, J., Diet, R., Jacobs, D., et al. (2000) DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immunol. 68, 38–45.
Martin, V., Supanitsky, A., Echeverria, P. C., Litwin, S., Tanos, T., de Roodt, A. R., et al. 2004. Recombinant Gra4 or Rop2 combined with alum or gra4 DNAa provides partial protection in chronic murine models of toxoplamosis. Clin. Diagn. Lab. Immunol. 11, 704–710.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clemente, M., Curilovic, R., Sassone, A. et al. Production of the main surface antigen of Toxoplasma gondii in tobacco leaves and analysis of its antigenicity and immunogenicity. Mol Biotechnol 30, 41–49 (2005). https://doi.org/10.1385/MB:30:1:041
Issue Date:
DOI: https://doi.org/10.1385/MB:30:1:041