Abstract
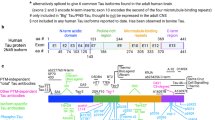
The pathological diagnosis of Alzheimer’s disease (AD) depends on the presence of plaques consisting of the β-amyloid peptide as well as neurofibrillary tangles consisting of paired helical filaments (PHFs) of the tau (τ) protein. The role of each type of pathology in the pathogenesis and progression of AD remains unclear. Previous hypotheses suggested that these two processes were independent, whereas more recent data suggest that there may be a bidirectional interaction between these two pathological processes.
The identification of the neurotoxic effects of β-amyloid and the discovery of mutations responsible for earlyonset Alzheimer’s disease (EOAD) and their linkage to β-amyloid overproduction, has made the amyloid hypothesis of AD the predominant influence for therapeutic targets. Several approaches have emerged from preclinical testing and have entered early phases of clinical developments.
The recent identification of τ mutations and their linkage to progressive neurodegenerative disorders provides a counterbalancing influence on the search for therapeutic targets for AD. Therapeutic approaches that are targeted to either β-amyloid or τ share certain features at the level of pharmacology and will face many of the same challenges as they progress through drug development paradigms. The aim of this article is to provide a brief overview of some of the commonalities and the challenges faced by τ-related therapeutic strategies. The issues discussed in this article are not exhaustively dealt with in either scope or detail.
Similar content being viewed by others
References
Billingsley M. L. and Kincaid R. L. (1997) Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem. J. 323 (Pt. 3), 577–591.
Brion J. P., Anderton B. H., et al. (2001) Neurofibrillary tangles and tau phosphorylation. Biochem. Soc. Symp. 67, 81–88.
Delaere P., Duyckaerts C., et al. (1989) Tau, paired helical filaments and amyloid in the neocortex: a morphometric study of 15 cases with graded intellectual status in aging and senile dementia of Alzheimer type. Acta Neuropathol. (Berl.) 77(6), 645–653.
Flaherty D. B., Soria J. P., et al. (2000) Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J. Neurosci. Res. 62(3), 463–472.
Guttmann R. P., Erickson A. C., et al. (1995) Tau self-association: stabilization with a chemical cross-linker and modulation by phosphorylation and oxidation state. J. Neurochem. 64(3), 1209–1215.
Hagestedt T., Lichtenberg B., et al. (1989) Tau protein becomes long and stiff upon phosphorylation: correlation between paracrystalline structure and degree of phosphorylation. J. Cell Biol. 109(4 Pt. 1), 1643–1651.
Lambert M. P., Viola K. L., et al. (2001) Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J. Neurochem. 79(3), 595–605.
Lewis J., Dickson D. W., et al. (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293(5534), 1487–1491.
Mack T. G., Dayanandan R., et al. (2001) Tau proteins with frontotemporal dementia—17 mutations have both altered expression levels and phosphorylation profiles in differentiated neuroblastoma cells. Neuroscience 108(4), 701–712.
Raskind M. A., Carta A., et al. (1995) Is early-onset Alzheimer disease a distinct subgroup within the Alzheimer disease population? Alzheimer Dis. Assoc. Disord. 9(Suppl. 1), S2-S6.
Steiner B., Mandelkow E. M., et al. (1990) Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 9(11), 3539–3544.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gold, M. Tau therapeutics for alzheimer’s disease. J Mol Neurosci 19, 329–334 (2002). https://doi.org/10.1385/JMN:19:3:329
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:19:3:329