Abstract
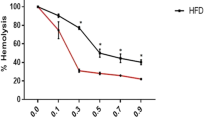
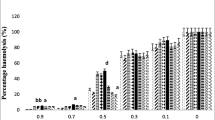
The present study was planned to explain the relation between erythrocyte osmotic fragility and oxidative stress and antioxidant statue in primary hypothyroid-induced experimental rats. Twenty-four Spraque Dawley type female rats were divided into two, as control (n=12) and experimental (n=12), groups weighing between 160 and 200 g. The experimental group animals have received tap water methimazole added standard fodder to block the iodine pumps for 30 d (75 mg/100 g). Control group animals were fed tap water and only standard fodder for the same period. At the end of 30 d blood samples were drawn from the abdominal aorta of the rats under ether anesthesia. T3, T4, and TSH levels were measured and the animals that had relatively lower T3, T4, and higher TSH levels were accepted as hypothyroid group. Hormone levels of the control group were at euthyroid conditions. Osmotic fragility, as a lipid peroxidation indicator malondialdehyde (MDA), antioxidant defense system indicators superoxide dismutase (SOD) and glutathione (GSH) levels were measured in the blood samples. Osmotic fragility test results: There was no statistically significant difference found between maximum osmotic hemolysis limit values of both group. Minimum osmotic hemolysis limit value of hypothyroid group was found to be higher than that of control group values (p<0.02). The standard hemolysis and hemolytic increment curve of the hypothyroid group drawn according to osmotic fragility test results was found to be shifted to the right when compared to control group’s curve. This situation and hemolytic increment value, which shows maximum hemolysis ratio, is the proof of increased osmotic fragility of the erythrocytes in hypothyroidism. There is no statistically significant difference found between hypothyroid and control groups in the lipid peroxidation indicator MDA and antioxidant indicators SOD and GSH levels. As a result of our study it may be concluded that hypothyroidism may lead to an increase in osmotic fragility of erythrocytes. But the increase in erythrocyte osmotic fragility does not originate from lipid peroxidation.
Similar content being viewed by others
References
Ansell, J. E. (1996). In: Werner and Ingbar’s the thyroid. Braverman, L. E. and Utiger, R. D. (eds.). Lippincott-Raven: Philadelphia, PA, pp. 821–825.
Jain, S. K., Mohandas, N., Clark, M. R., and Shobel, S. B. (1983). Br. J. Haematol. 53, 247–252.
Sies, H. (1985). In: Oxidative stress. Sies, H. (ed.). Academic Press: Orlando, FL.
Wills, E. D. (1985). In: The role of dietary components in oxidative stress in tissues. Sies, H. (ed.). Academic Press: Orlando, FL.
Hubel, C. A., Griggs, K. C., and McLaughlin, M. K. (1989). Am. J. Physiol. 256, H1539-H1545.
van Ginkel, G. and Sevanian, A. (1994). Methods Enzymol. 233, 273–288.
Aguirre, F., Martin, I., Grinspon, D., et al. (1998). Free Rad. Biol. Med. 24, 580–585.
Hebbel, R. P. (1986). J. Lab. Clin. Med. 107, 401–404.
Brzezinska, S. (2001). Acta Vet. Hung. 49, 413–419.
Devasena, T., Lalitha, S., and Padma, K. (2001). Clin. Chim. Acta 308, 155–161.
Asayama, K., Dobashi, K., Hayashibe, H., Megata, Y., and Kato, K. (1987). Endocrinol. 121, 2112–2118.
Seymen, H. O., Seven, A., Civelek, S., et al. (1999). J. Basic Clin. Physiol. Pharmacol. 10, 315–325.
Venditti, P., Balestrieri, M., Di Meo, S., and De Leo, T. (1997). J. Endocrinol. 155, 151–157.
Krishnamurthy, S. and Prasanna, D. (1984). Acta Vitaminol. Enzymol. 6, 17–21.
Dumitriu, L., Bartoc, R., Ursu, H., Purice, M., and Ionescu, V. (1988). Endocrinologie 26, 35–38.
Swaroop, A. and Ramasarma, T. (1985). Biochem. J. 226, 403–408.
Paller, M. S. (1986). Kidney International 29, 1162–1166.
Mano, T., Sinohara, R., Sawai, Y., et al. (1995). J. Endocrinol. 147, 361–365.
Pereira, B., Rosa, L. F., Safi, D. A., Bechara, E. J., and Curi, R. (1994). J. Endocrinol. 140, 73–77.
Venditti, P., De Rosa, R., and Di Meo, S. (2003). Free Radical Biology Medicine 35(5), 485–494.
Ghosh, S., Rahaman, S. O., and Sarkar, P. K. (1999). Neuro. Report 10, 2361–2365.
Rahaman, S. O., Ghosh, S., Mohanakumar, K. P., Das, S., and Sarkar, P. K. (2001). Neurosci. Res. 40, 273–279.
Sahun, M., Villabona, C., Rosel, P., et al. (2001). J. Endocrinol. 168(3), 435–445.
Bhatacharyya, A. D. and Dash R. J. (1994). J. Assoc. Physicians India. 42(5), 366–368.
Dariyerli, N., Andican, G., Çatakoglu, A. B., Hatemi, H., and Burçak, G. (2003). Tohoku J. Exp. Med. 199, 59–68.
Suess, J., Limenton, D., Dameshek, W., and Dolloft, J. M. A. (1954). Blood 3, 1250–1303.
Slater, T. F. (1984). Methods Enzymol. 105, 283–293.
Anderson, M. E. (1989). In: Enzymatic and chemical methods for the determination of glutathione; glutathione: chemical, biochemical and medical aspects. Vol. A. Dolphin, D., Poulson, R., and Avramovic, O. (eds.). Wiley: New York.
Nebot, C., Moutet, M., Huet, P., Xu, J. Z., Yadan, J. C., and Chaudiere, J. (1993). Anal. Biochem. 214, 442–451.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dariyerli, N., Toplan, S., Akyolcu, M.C. et al. Erythrocyte osmotic fragility and oxidative stress in experimental hypothyroidism. Endocr 25, 1–5 (2004). https://doi.org/10.1385/ENDO:25:1:01
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ENDO:25:1:01