Abstract
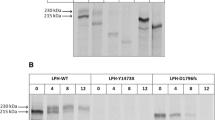
Glucose-galactose malabsorption (GGM) is an autosomal recessive disease that presents in newborn infants as a life-threatening diarrhea. The diarrhea ceases within 1 h of removing oral intake of lactose, glucose, and galactose, but promptly returns with the introduction of one or more of the offending sugars into the diet. Our goal is to determine whether or not mutations in the sodium-glucose cotransporter gene (SGLT1) are responsible for GGM. We first isolated the human cDNA (hSGLT1), mapped the gene and identified its chromosomal location (22q13.1). Our approach was then to screen GGM patients for mutations in hSGLT1 and then determine if these caused defects, in sugar transport using the Xenopus laevis oocyte expression system. In 46 patients we have identified the mutations responsible for GGM. These included missense, nonsense, frame shift, splice site, and promoter mutations. In 30 patients, the same mutations were on both alleles, and the remaining 16 had different mutations on each allele (compound heterozygotes). Several mutations (e.g., C355S) were found in unrelated patients. The nonsense, frame shift, and splice site mutations all produce nonfunctional truncated proteins. In 22 out of the 23 missense mutations tested in the oocyte expression system, the proteins were translated and were stable in the cell, but did not reach the plasma membrane. In four of these mutants, an alanine residue was replaced by a valine, and in two, the trafficking defect was rescued by changing the valine to cysteine. One mutant protein (Q457R) did reach the plasma membrane, but it was unable to transport the sugar across the cell membrane. We conclude that mutations in the SGLT1 gene are the cause of glucose-galactose malabsorption, and sugar transport is impaired mainly because the mutant proteins are either truncated or are not targeted properly to the cell membrane.
Similar content being viewed by others
References
Wright, E. M. (1998) Glucose galactose malabsorption. Am. J. Physiol.: Gastrointest. Liver Physiol. 275, G879-G882.
Wright, E. M., Martín, G. M., and Turk, E. (2001) Familial glucose-galactose malabsorption and hereditary renal glycosuria, in Metabolic Basis of Inherited Disease, 8th ed. (Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle, D., eds.) McGraw Hill, NY, Vol. III, pp. 4891–4908.
Laplane, R., Polonovski, C., Etienne, M., Debray, P., Lods, J. C., and Pissarro, B. (1962) L'intolerance aux sucres, a transfert intestinal actif. Arch. Fr. Pediatr. 19, 895–944.
Lindquist, B. and Meeuwisse, G. W. (1962) Chronic diarrhoea caused by monosaccharide malabsorption. Acta Paediatr. 51, 674–685.
Meeuwisse, G. W. and Melin, K. (1969) Studies in glucose-galactose malabsorption. A clinical study of 6 cases and a genetic study. Acta Paediat. Scand. S188, 3–19.
Schneider, A. J., Kinter, W. B., and Stirling, C. E. (1966) Glucose-galactose malabsorption: report of a case with autoradiographic studies of a mucosal biopsy. N. Engl. J. Med. 274, 305–312.
Stirling, C. E., Schneider, A. J., Wong, M. D., and Kinter, W. B. (1972) Quantitative radioautography of sugar transport in intestinal biopsies from normal humans and a patient with glucose-galactose malabsorption. J. Clin. Invest. 51, 438–451.
Meeuwisse, G. W. and Dahlqvist, A. (1969) Glucose-galactose malabsorption. A study with biopsy of the small intestine. Acta Paediat. Scand. 57, 273–280.
Turk, E. and Wright, E. M. (1997) Membrane topological motifs in the SGLT cotransporter family. J. Membr. Biol. 159, 1–20.
Loo, D. D. F., Hirayama, B. A., Gallardo, E. M., Lam, J. T., Turk, E., and Wright, E. M. (1998) Conformational changes couple Na+ and glucose transport. PNAS 95, 7789–7794.
Wright, E. M. (2001) Renal Na+/glucose cotransporters. Am. J. Physiol: Renal Physiol. 280, F10-F18.
Martin, G. M., Turk, E., Lostao, M. P., Kerner, C., and Wright, E. M. (1996) Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nature Genet. 12, 216–220.
Kasahara, M., Maeda, M., Hayashi, S., Mori, Y., and Toshiaki, A. (2001) A missense mutation in the Na+/glucose cotransporter gene SGLT1 in a patient with congenital glucose-galactose malabsorption: normal trafficking but inactivation of the mutant protein. Biochim. Biophys. Acta 1536, 141–147.
Turk, E., Zabel, B., Mundlos, S., Dyer, J., and Wright, E. M. (1991) Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 350, 354–356.
Martín, M. G., Lostao, M. P., Turk, E., Lam, J., Kreman, M., and Wright, E. M. (1997) Compound missense mutations in the sodium/D-glucose cotransporter (SGLT1) results in trafficking defects. Gastroenterology 112, 1206–1212.
Lam, J. T., Martín, M. G., Turk, E., Bosshard, N. U., Steinmann, B., and Wright, E. M. (1999) Missense mutations in SGLT1 cause glucose-galactose malabsorption by trafficking defects. Biochim. Biophys. Acta 1453, 297–303.
Zampighi, G. A., Kreman, M., Boorer, K. J., Loo, D. D. F., Bezanilla, F., Chando, G., et al. (1995) A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J. Membr. Biol. 148, 65–78.
Diez-Sampedro, A., Wright, E. M., and Hirayama, B. A. (2001) Residue 457 controls sugar binding and transport in the Na+/glucose cotransporter. J. Biol. Chem. 276(52), 49,188–49,194.
Panajotova-Heiermann, M., Loo, D. D. F., Klong, C.-T., Lever, J. E., and Wright, E. M. (1996) Sugar binding to Na+/glucose cotransporters is determined by the C-terminal half of the protein. J. Biol. Chem. 271, 10,029–10,034.
Panayotova-Heiermann, M., Eskandari, S., Zampighi, G. A., and Wright, E. M. (1997) Five transmembrane helices form the sugar pathway through the Na+/glucose transporter. J. Biol. Chem. 272, 20,324–20,327.
Panayotova-Heiermann, M., Leung, D. W., Hirayama, B. A., and Wright, E. M. (1999) Purification and functional reconstitution of a truncated human Na+/glucose cotransporter (SGLT1) expressed in E. coli. FEBS Lett. 459, 386–390.
Montes, R. G., Gottal, R. F., Bayless, T. M., Hendrix, T. R., and Perman, J. A. (1992) Breath hydrogen testing as a physiology laboratory exercise for medical students. Am. J. Physiol. 262, S25-S28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, E.M., Turk, E. & Martin, M.G. Molecular basis for glucose-galactose malabsorption. Cell Biochem Biophys 36, 115–121 (2002). https://doi.org/10.1385/CBB:36:2-3:115
Issue Date:
DOI: https://doi.org/10.1385/CBB:36:2-3:115