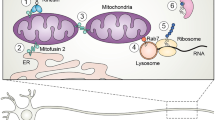
Abstract
Charcot-Marie-Tooth disease (CMT) is a clinically and genetically heterogeneous group of disorders and is the most common inherited neuromuscular disorder, with an estimated overall prevalence of 17–40/10.000. Although there has been major advances in the understanding of the genetic basis of CMT in recent years, the most useful classification is still a neurophysiological classification that divides CMT into type 1 (demyelinating, median motor conduction velocity <38 m/s) and type 2 (axonal; median motor conduction velocity> 38 m/s). An intermediate type is also increasingly being described. Inheritance can be autosomal-dominant (AD), X-linked, or autosomal-recessive (AR). ADCMT1 is the most common type of CMT and was the first form of CMT in which a causative gene was described. This review provides an up-to-date overview of AD CMT1 concentrating on the molecular genetics as the clinical, neurophysiological, and pathological features have been covered elsewhere. Four genes (PMP22, MPZ, LITAF, and EGR2) have been described in the last 15 yr associated with AD CMTI and a further gene (NEFL), originally described as causing AD CMT2 can also cause AD CMT1 (by neurophysiological criteria) (Table 1, Figs. 1, and 2). Studies have shown many of these genes, when mutated, can cause a wide range of CMT phenotypes from the relatively mild CMT1 to the more severe Dejerine-Sottas disease and congenital hypomyelinating neuropathy, and even in some cases axonal CMT2 (Table 1). This review discusses what is known about these genes and in particular how they cause a peripheral neuropathy, when mutated.
Similar content being viewed by others
References
Abe K. T., Lino A. M., Hirata M. T., et al. (2004) A novel stop codon mutation in the PMP22 gene associated with a variable phenotype. Neuromuscul. Disord. 14(5), 313–320.
Adlkofer K., Frei R. Neuberg D. H., Zielasek J. Toyka K. V., and Suter U. (1997a) Heterozygous peripheral myelin protein 22-deficient mice are affected by a progressive demyelinating tomaculous neuropathy. J. Neurosci. 17(12), 4662–4671.
Adlkofer K. Martini R., Aguzzi A. Zielasek J., Toyka K. V., and Suter U. (1995) Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat. Genet. 11(3), 274–280.
Adlkofer K., Naef R., and Suter U. (1997b) Analysis of compound heterozygous mice reveals that the Trembler mutation can behave as a gain-of-function allele. J. Neurosci. Res., 49(6), 671–680.
Bellone E., Di Maria E., Soriani S. et al. (1999) A novel mutation (D305V) in the early growth response 2 gene is associated with severe Charcot-Marie-Tooth type 1 disease. Hum. Mutat. 14(4), 353–354.
Bennett C. L., Shirk A. J., Huynh H. M., Street V. A., et al. (2004) SIMPLE mutation in demyelinating neuropathy and distribution in sciatic nerve. Ann. Neurol.. 55(5), 713–720.
Berger P., Young P., and Suter U. (2002) Molecular cell biology of Charcot-Marie-Tooth disease. Neurogenetics 4(1), 1–15.
Bird T. D., Ott J., and Giblett E. R. (1982) Evidence for linkage of Charcot-Marie-Tooth neuropathy to the Duffy locus on chromosome 1. Am. J. Hum. Genet. 34(3), 388–394.
Boerkoel C. F., Inoue K., Reiter L. T., Warner L. E., and Lupski J. R. (1999) Molecular mechanisms for CMT1A duplication and HNPP deletion. Ann. NY Acad. Sci. 883, 22–35.
Boerkoel C. F., Takashima H. Bacino C. A., Daentl D., and Lupski J. R. (2001) EGR2 mutation R359W causes a spectrum of Dejerine-Sottas neuropathy. Neurogenetics. 3(3), 153–157.
Boerkoel C. F., Takashima H., Garcia C. A., et al. (2002) Charcot-Marie-Tooth disease and related neuropathies: mutation distribution and genotype-phenotype correlation. Ann. Neurol.. 51(2), 190–201.
Brancolini C., Edomi P., Marzinotto S., and Schneider C. (2000). Exposure at the cell surface is required for gas3/PMP22 To regulate both cell death and cell spreading: implication for the Charcot-Marie-Tooth type 1A and Dejerine-Sottas diseases. Mol. Biol. Cell.. 11(9), 2901–2914.
Brownlees J., Ackerley S., Grierson A. J. et al. (2002) Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum. Mol. Genet.. 11(23), 2837–2844.
Chance P. F., Alderson M. K., Leppig K. A., et al. (1993) DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 72(1), 143–151.
Chance P. F., Bird T. D., O'Connell P., Lipe H., Lalouel J. M., Leppert M. (1990) Genetic linkage and heterogeneity in type I Charcot-Marie-Tooth disease (hereditary motor and sensory neuropathy type I). Am. J. Hum. Genet.. 47(6), 915–925.
Chapon F., Latour P., Diraison P., Schaeffer S., Vandenberghe A. (1999) Axonal phenotype of Charcot-Marie-Tooth disease associated with a mutation in the myelin protein zerogene. J. Neurol. Neurosurg. Psychiatry. 66(6), 779–782.
Chavrier P., Janssen-Timmen U., Mattei MG., Zerial M., Bravo R., and Charnay P. (1989) Structure, chromosome location, and expression of the mouse zinc finger gene Krox-20: multiple gene products and coregulation with the proto-oncogene c-fos. Mol. Cell Biol.. 9(2), 787–797.
Choi B. O., Lee M. S., Shin S. H., et al. (2004) Mutational analysis of PMP22, MPZ, GJB1, EGR2 and NEFL in Korean Charcot-Marie-Tooth neuropathy patients. Hum. Mutat. 24(2), 185–186.
De Angelis M. V., Di Muzio A., Capasso M., et al. (2004) Segmental conduction abnormalities and myelin thickenings in Val102/fs null mutation of MPZ gene. Neurology. 63(11), 2180–2183.
De Jonghe P., Mersivanova I. Nelis E., et al. (2001) Further evidence that neurofilament light chain gene mutations can cause Charcot-Marie-Tooth disease type 2E. Ann. Neurol.. 49(2), 245–249.
De Jonghe P., Timmerman V., Ceuterick C., et al. (1999) The Thr124Met mutation in the peripheral myelin protein zero (MPZ) gene is associated with a clinically distinct Charcot-Marie-Tooth phenotype. Brain. 122(Part 2), 281–290.
De Jonghe P., Timmerman V., Nelis E., Martin J. J., and Van Broeckhoven C. (1997) Charcot-Marie-Tooth disease and related peripheral neuropathies. J. Peripher. Nerv. Syst.. 2(4), 370–387.
Donaghy M., Sisodiya S. M., Kennett R., McDonald B., Haites N., and Bell C. (2000) Steroid responsive polyneuropathy in a family with a novel myelin protein zero mutation. J. Neurol. Neurosurg. Psychiatry 69(6), 799–805.
Dubourg O., Tardieu S., Birouk N., et al. (2001) The frequency of 17p11.2 duplication and Connexin 32 mutations in 282 Charcot-Marie-Tooth families in relation to the mode of inheritance and motor nerve conduction velocity. Neuromuscul. Disord. 11(5), 458–463.
D'Urso D., Brophy P. J., Staugaitis S. M., et al. (1990) Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron. 4(3), 449–460.
D'Urso D., Ehrhardt P., and Muller H. W. (1999) Peripheral myelin protein 22 and protein zero: a novel association in peripheral nervous system myelin. J. Neurosci. 19(9), 3396–3403.
D'Urso D., and Muller H. W. (1997) Insand outs of peripheral myelin protein-22; mapping transmembrane topology and intracellular sorting. J. Neurosci. Res. 49(5), 551–562.
D'Urso D., Prior R., Greiner-Petter R., Gabreels-Festen A. A., and Muller H. W. (1998) Overloaded endoplasmic reticulum-Golgi compartments a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22. J. Neurosci.. 18(2), 731–740.
D'Urso D., Schmalenbach C., Zoidl G., Prior R., and Muller H. W. (1997) Studies on the effects of altered PMP22 expression during myelination in vitro. J. Neurosci. Res.. 48(1), 31–42.
Fabrizi G. M., Cavallaro T., Angiari C., et al. (2004) Giant axon and neurofilament accumulation in Charcot-Marie-Tooth disease type 2E. Neurology 62(8), 1429–1431.
Fabrizi G. M., Ferrarini M., Cavallaro T., Jarre L., Polo A., and Rizzuto N. (2001) A somatic and germline mosaic mutation in MPZ/P(0) mimics recessive inheritance of CMT1B Neurology. 57(1), 101–105.
Filbin M. T., Walsh F. S., Trapp B. D., Pizzey J. A., and Tennekoon G. I. (1990) Role of myelin P0 protein as a homophilic adhesion molecule Nature. 344(6269), 871–872.
Filbin M. T., Zhang K., Li W., and Gao Y. (1999) Characterization of the effect on adhesion of different mutations in myelin P0 protein Ann. NY Acad. Sci. 883, 160–167.
Fortun J., Dunn W. A. Jr., Joy S., Li J., and Notterpek L. (2003) Emerging role for autophagy in the removal of aggresomes in Schwann cells J. Neurosci. 23(33), 10,672–10,680.
Fortun J., Li J., Go J., Fenstermaker A., Fletcher B. S., and Notterpek L. (2005) Impaired proteasome activity and accumulation of ubiquitinated substrates in a hereditary neuropathy model. J. Neurochem. 92(6), 1531–1541.
Gabreels-Festen A. A., Hoogendijk J. E., Meijerink P. H., et al. (1996) Two divergent types of nerve pathology in patients with different P0 mutations in Charcot-Marie-Tooth disease. Neurology 47(3), 761–765.
Georgiou D. M., Zidar J., Korosec M., Middleton L. T., Kyriakides T., and Christodoulou K. (2002) A novel NF-L mutation Pro22Ser is associated with CMT2 in a large Slovenian family. Neurogenetics 4(2), 93–96.
Giambonini-Brugnoli G., Buchstaller J., Sommer L., Suter U., and Mantei N. (2005) Distinct disease mechanisms in peripheral neuropathies due to altered peripheral myelin protein 22 gene dosage or a Pmp22 point mulation. Neurobiol. Dis. 18(3), 656–668.
Giese K. P., Martini R., Lemke G., Soriano P., and Schachner M. (1992) Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell 71(4), 565–576.
Ginsberg L., Malik O., Kenton A. R., et al. (2003) Coexistent hereditary and inflammatory neuropathy. Brain. 127(1), 193–202.
Hanemann C. O., Gabreels-Festen A. A., Stoll G., and Muller H. W. (1997) Schwann cell differentiation in Charcot-Marie-Tooth disease type 1A (CMT1A): normal number of myelinating Schwann cells in young CMT1A patients and neural cell adhesion molecule expression in onion bulbs. Acta Neuropathol. (Berl.) 94(4), 310–315.
Harati Y. and Butler I. (1985) Congenital hypomyelinating neuropathy. J. Neurol. 48(12), 1269–1276.
Hayasaka K., Himoro M., Sato W., et al. (1993a) Charcot-Marie-Tooth neuropathy type 1B is associated with mutations of the myelin P0 gene. Nat. Genet. 5(1), 31–34.
Hayasaka K., Himoro M., Sawaishi Y., et al. (1993b) De novo mutation of the myelin P0 gene in Dejerine-Sottas disease (hereditary motor and sensory neuropathy type III). Nat. Genet. 5(3), 266–268.
Inoue K., Dewar K., Katsanis N., et al. (2001) The 1.4-Mb CMT1A duplication/HNPP deletion genomic region reveals unique genome architectural features and provides insights into the recent evolution of new genes. Genome Res. 11(6) 1018–1033.
Ionasescu V. V., Ionasescu R., Searby C., and Barker D. F. (1993) Charcot-Marie-Tooth neuropathy type 1A with both duplication and non-duplication. Hum. Mol. Genet. 2(4), 405–410.
Ionasescu V. V., Searby C. C., Ionasescu R., Chatkupt S., Patel N., and Koenigsberger R. (1997) Dejerine-Sottas neuropathy in mother and son with same point mutation of PMP22 gene. Muscle Nerve 20(1), 97–99.
Isaacs A. M., Davies K. E., Hunter A. J., et al. (2000) Identification of two new Pmp22 mouse mutants using large-scale mutagenesis and a novel rapid mapping strategy. Hum. Mol. Genet. 9(12), 1865–1871.
Jordanova A., De Jonghe P., Boerkoel C. F., et al. (2003) Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot-Marie-Tooth disease. Brain 126(Part 3), 590–597.
Julian B. A., Phillips J. A. 3rd, Orlando P. J., Wyatt R. J., and Butler M. G. (1987) Analysis of immunoglobulin heavy chain restriction fragment length polymorphisms in IgA nephropathy. Semin. Nephrol. 7(4), 306–310.
King P. H., Waldrop R., Lupski J. R., and Shaffer L. G. (1998) Charcot-Marie-Tooth phenotype produced by a duplicated PMP22 gene as part of a 17p trisomy-translocation to the X chromosome. Clin. Genet. 54(5) 413–416.
Kiyosawa H. and Chance P. F. (1996). Primate origin of the CMT1A-REP repeat and analysis of a putative transposon-associated recombinational hotspot. Hum. Mol. Genet. 5(6), 745–753.
Klein C. and Dyck P. (2005) Molecular Genetics of HMSN II (CMT 2), in Peripheral Neuropathy 2005, 4th ed., Dyck, P.J. and Thomas P.K. eds., Elsevier Saunders, Philadelphia, pp. 1717–1751.
Krajewski K. M., Lewis R. A., Fuerst D. R., et al. (2000) Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain 123(Part 7), 1516–1527.
Latour P., Gatignol A., Boutrand L., et al. (1999) A R381H mutation in the EGR2 gene associated with a severe peripheral neuropathy with hypotonia. J. Periph. Nerv. Syst. 4, 293–294.
Le N., Nagarajan R., Wang, J. Y., Araki T., Schmidt R.E., and Milbrandt J. (2005a) Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc. Natl. Acad. Sci. USA 102(7)., 2569–2601.
Le N., Nagarajan R., Wang, J. Y., et al. (2005b) Nab proteins are essential for peripheral nervous system myelination. Nat. Neurosci. 8(7), 932–940.
Leblanc S. E., Srinivasan R., Ferri C., et al. (2005). Regulation of cholesterol/lipid biosynthetic genes by Egr2/Kro×20 during peripheral nerve myelination. J. Neurochem. 93(3),737–748.
LeGuern E., Gouider R., Mabin D., et al. (1997). Patients homozygous for the 17p11.2 duplication in Charcot-Marie-Tooth type 1A disease. Ann. Neurol. 41(1), 104–108.
Lenssen P. P., Gabreels-Festen A.A., Valentijn L. J., et al. (1998) Hereditary neuropathy with liability to pressure palsies. Phenotypic differences between patients with the common deletion and a PMP22 frame shift mutation. Brain 121(Part 8), 1451–1458.
Liu Q, Xie F, Siedlak S. L., et al. (2004) Neurofilament proteins in neurodegenerative diseases. Cell Mol. Life Sci. 61(24), 3057–3075.
Lupski J. and Chance P. (2005) Hereditary Motor and Sensory Neuropathies Involving Altered Dosage or Mutation of PMP22. The CMT1A Duplication and HNPP Deletion, in Peripheral Neuropathy, 4th ed., Dyck, P. J. and Thomas P. K. eds., Elseiver Saunders, Philadelphia, pp. 1659–1680.
Lupski J. R. (2000) A xonal Charcot-Marie-Tooth disease and the neurofilament light gene (NF-L). Am. J. Hum. Genet. 67(1), 8–10.
Lupski J. R., de Oca-Luna R. M., Slaugenhaupt, S., et al. (1991) DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell 66(2) 219–232.
Magyar J. P., Martini R., Ruelicke T., et al. (1996) Impaired differentiation of Schwann cells in transgenic mice with increased PMP22 gene dosage. J. Neurosci. 16(17) 5351–5360.
Marques W Jr., Sweeney M. G., and Wood N. W. (2003) Thr(118) Met amino acid substitution in the peripheral myelin protein 22 does not influence the clinical phenotype of Charcot-Marie-Tooth disease type 1A due to the 17p11.2-p12 duplication. Braz. J. Med. Biol. Res. 36(10), 1403–1407.
Marrosu M. G., Vaccargiu S., Marrosu G., Vannelli A., Cianchetti C., and Muntoni F. (1998) Charcot-Marie-Tooth disease type 2 associated with mutation of the myelin protein zero gene. Neurology 50(5), 1397–1401.
Martini R., Zielasek J., Toyka K. V., Giese K. P., and Schachner M. (1995) Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nat. Genet. 11(3), 281–286.
Meggouh F., de Visser M., Arts W. F., De Coo R. I., van Schaik I. N., and Baas F. (2005) Early onset neuropathy in a compound form of Charcot-Marie-Tooth disease. Ann. Neurol. 57(4), 589–591.
Malcangi R. C., Magnaghi V., Cavarretta I., et al. (1999a) Progesterone derivatives are able to influence peripheral myelin protein 22 and P0 gene expression: possible mechanisms of action. J. Neurosci. Res. 56(4), 349–357.
Melcangi R. C., Magnaghi V., Martini L. (1999b) Steroid metabolism and effects in central and peripheral glial cells. J. Neurobiol. 40(4), 471–483.
Menichella D. M., Arroyo E. J., Awatramani R., et al. (2001) Protein zero is necessary for E-cadherin-mediated adherens junction formation in Schwann cells. Mol. Cell Neurosci. 18(6), 606–618.
Mersiyanova I. V., Ismailov S. M., Polyakov A. V., et al. (2000a) Screening for mutations in the peripheral myelin gene PMP22,MPZ and C×32 (GJB1) in Russian Charcot-marie-Tooth neuropathy patients. Hum. Mutat. 15(4), 340–347.
Mersiyanova I. V., Perepelov A. V., Polyakov A. V., et al. (2000b) A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am. J. Hum. Genet. 67(1), 37–46.
Meuleman J., Pou-Serradell A., Lofgren A., et al. (2001) A novel 3′-splice site mutation in peripheral myelin protein 22 causing hereditary neuropathy with liability to pressure palsies. Neuromuscul. Disord. 11(4) 400–403.
Mirsky R. and Jessen K. R. (1999) The neurobiology of Schwann cells. Brain. Pathol. 9(2), 293–311.
Moriwaki Y., Begum N. A., Kobayashi M., Matsumoto M., Toyoshima K., and Seya T. (2001) Mycobacterium bovis Bacillus Calmette-Guerin and its cell wall complex induce a novel lysosomal membrane protein, SIMPLE, that bridges the missing link between lipopolysaccharide and p53-inducible gene, LITAF(PIG7), and estrogen-inducible gene, EET-1. J. Biol. Chem. 276(25), 23,065–23,076.
Mostacciuolo M. L., Righetti E., Zortea M., et al. (2001) Charcot-Marie-Tooth disease type I and related demyelinating neuropathies: Mutation analysis in a large cohort of Italian families. Hum. Mutat. 18(1), 32–41.
Myokai F., Takashiba S., Lebo R., and Amar S. (1999) A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor alpha gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc. Natl. Acad. Sci. USA 96(8), 4518–4523.
Naef R., Adlkofer K., Lescher B., and Suter U. (1997) Aberrant protein trafficking in Trembler suggests a disease mechanism for hereditary human peripheral neuropathies. Mol. Cell. Neurosci. 9(1), 13–25.
Naef R. and Suter U. (1998) Many facets of the peripheral myelin protein PMP22 in myelination and disease. Microsc. Res. Tech. 41(5), 359–371.
Nagarajan R., Svaren J., Le N., Araki T., Watson M., and Milbrandt J. (2001). EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron 30(2), 355–368.
Nelis E., Haites N., and Van Broeckhoven C. (1999) Mutations in the peripheral myelin genes and associated genes in inherited peripheral neuropathies. Hum. Mutat. 13(1), 11–28.
Nelis E., Holmberg B., Adolfsson R., Holmgren G., and van Broeckhoven C. (1997) PMP22 Thr(118)Met: recessive CMT1 mutation or polymorphims? Nat. Genet. 15(1), 13–14.
Nelis E., Van Broeckhoven C., De Jonghe P., et al. (1996) Estimation of the mutation frequencies in Charcot-Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressurepalsies: a European collaborative study. Eur. J. Hum. Genet. 4(1), 25–33.
Notterpek, L., Ryan M. C., Tobler A. R., and Shooter E. M. (1999) PMP22 accumulation in aggresomes: implications for CMT1A pathology. Neurobiol. Dis. 6(5), 450–460.
Numakura C., Lin C., Oka, N., Akiguchi I., and Hayasaka K. (2000) Hemizy gous mutation of the peripheral myelin protein 22 gene associated with Charcot-Marie-Tooth disease type 1. Ann. Neurol. 47(1), 101–103.
Numakura, C., Shirahata E., Yamashita S., et al. (2003) Screening of the early growth response 2 gene in Japanese patients with Charcot-Marie-Tooth disease type 1. J. Neurol. Sci. 210(1,2), 61–64.
Palau F., Lofgren A., De Jonghe P., et al. (1993) Origin of the de novo duplication in Charcot-Marie-Tooth disease type 1A: unequal nonsister chromatid exchange during spermatogenesis. Hum. Mol. Genet. 2(12), 12031–2035.
Pareyson D., Taroni F., Botti S., et al. (2000) Cranial nerve involvement in CMT disease type 1 due to early growth response 2 gene mutation. Neurology. 54(8), 1696–1698.
Parman Y., Plante-Bordeneuve V., Guiochon-Mantel A., Eraksoy M., and Said G. (1999) Recessive inheritance of a new point mutation of the PMP22 gene in Dejerine-Sottas disease. Ann. Neurol. 45(4), 518–522.
Passage E., Norreel J. C., Noack-Fraissignes P., et al. (2004) Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat. Med. 10(4),396–401.
Pentao L., Wise C. A., Chinault A. C., Patel P. I., and Lupski J. R. (1992) Charcot-Marie-Tooth type 1A duplication appears to arise from recombination at repeat sequences flanking the 1.5 Mb monomer unit. Nat. Genet. 2(4), 292–300.
Perea J., Robertson A., Tolmachova T., et al. (2001) Induced myelination and demyelination in a conditional mouse model of Charcot-Marie-Tooth disease type 1A. Hum. Mol. Genet. 10(10), 1007–1018.
Polyak K., Xia Y., Zweier J. L., Kinzler K. W., and Vogelstein B. (1997). A model for p53-induced apoptosis. Nature 389, (6648), 300–305.
Raeymaekers P., Timmerman V., De Jonghe P. et al. (1989) Localization of the mutation in an extended family with Charcot-Marie-Tooth neuropathy (HMSN I). Am. J. Hum. Genet. 45(6), 953–958.
Raeymaekers P., Timmerman V., Nelis E., et al. (1991) Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1A (CMT 1a). The HMSN Collaborative Research Group. Neuromuscul. Disord. 1(2), 93–97.
Reilly M. M. (2000) Classification of the hereditary motor and sensory neuropathies. Curr. Opin. Neurol. 13(5), 561–564.
Reiter L. T., Murakami T., Koeuth T., et al. (1996) A recombination hot spot responsible for two inherited peripheral neuropathies is located near a mariner transposon-like element. Nat. Genet. 12(3), 288–297.
Roa B. B., Garcia C. A., Pentao L., et al., (1993) Evidence for a recessive PMP22 point mutation in Charcot-Marie-Tooth disease type 1A. Nat. Genet. 5(2), 189–194.
Ryan M. C., Shooter E. M., and Notterpek L. (2002) Aggresome formation in neuropathy models based on peripheral myelin protein 22 mutations. Neurobiol. Dis. 10(2),109–118.
Saifi G. M., Szigeti K., Wiszniewski W., et al. (2005) SIMPLE mutations in Charcot-Marie-Tooth disease and the potential role of its protein product in protein degradation. Hum. Mutat. 25(4), 372–383.
Sancho S., Magyar J. P., Aguzzi A., and Suter U. (1999) Distalaxonopathy in peripheral nerves of PMP22-mutant mice. Brain 122(Part 8), 1563–1577.
Schmid C. D., Stienekemeier M., Oehen S., et al. (2000) Immune deficiency in mouse models for inherited peripheral neuropathies leads to improved myelin maintenance. J. Neurosci. 20(2), 729–735.
Schneider-Maunoury S., Topilko P., Seitandou T., et al. (1993) Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell 75(6), 1199–1214.
Senderek J., Hermanns B., Lehmann U., et al. (2000) Charcot-Marie-Tooth neuropathy type 2 and P0 point mutations: two novel amino acid substitutions (Asp61Gly; Tyr119Cys) and a possible “hotspot” on Thr 124Met. Brain Pathol. 10(2), 235–248.
Sereda M., Griffiths I., Puhlhofer A., et al. (1996) A transgenic rat model of Charcot-Marie-Tooth disease. Neuron 16(5), 1049–1060.
Sereda M. W., Meyer Zu Horste G., Suter U., Uzma N. and Nave K. A. (2003) Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A) Nat. Med. 4(12), 1533–1537.
Shames I., Fraser A., Colby J., Orfali W., and Snipes G. J. (2003) Phenotypic differences between peripheral myelin protein-22 (PMP22) and myelin protein zero (P0) mutations associated with Charcot-Marie-Tooth-related diseases J. Neuropathol. Exp. Neurol. 62(7), 751–764.
Shapiro L., Doyle J. P., Hensley P., Colman D. R., and Hendrickson W. A. (1996) Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 17(3), 435–449.
Shy M., Lupski J., Chance P., and Klein C.P.J.D. (2005) Hereditary Motor and Sensory Neuropathies: An overview of Clinical, Genetic, Electrophysiologic, and Pathologic Features, in Peripheral Neuropathy, 4th ed., Dyck, P.J. and Thomas P.K., eds., Elsevier Saunders, Philadelphia, pp.1681–1706.
Shy M. E., Jani A., Krajewski K., et al. (2004) Phenotypic clustering in MPZ mutations. Brain 127(Part 2), 371–384.
Snipes G. J., Suter U., Welcher A. A., and Shooter E. M. (1992) Characterization of a novel peripheral nervous system myelin protein (PMP-22/SR13). J. Cell Biol. 117(1), 225–238.
Sorour E., Thompson P., MacMillan J., and Upadhyaya M. (1995) Inheritance of CMT1A duplication from a mosaic father. J. Med. Genet. 32(6), 483–485.
Street V. A., Bennett C. L., Goldy J. D., et al. (2003) Mutation of a putative protein degradation gene LITAF/SIMPLE in Charcot-Marie-Tooth disease 1C. Neurology 60(1), 22–26.
Street V. A., Goldy J. D., Golden A. S., Tempel B. L., Bird T. D., and Chance P. F. (2002) Mapping of Charcot-Marie-Tooth disease type 1C to chromosome 16p identifies a novel locus for demyelinating neuropathies. Am. J. Hum. Genet. 70(1), 244–250.
Suh J. G., Ichihara N., Saigoh K., et al. (1997) An inframe deletion in peripheral myelin protein-22 gene causes hypomyelination and cell death of the Schwann cells in the new Trembler mutant mice. Neuroscience 79(3), 735–744.
Suter U., Moskow J. J., Welcher A. A., et al. (1992a) A leucine-to-proline mutation in the putative first transmembrane domain of the 22-kDa peripheral myelin protein in the trembler-J mouse. Proc. Natl. Acad. Sci. USA 89(10), 4382–4386.
Suter U., Welcher A. A., Ozcelik T., et al. (1992b) Trembler mouse carries a point mutation in a myelin gene. Nature 356(63–66), 241–244.
Takashima H., Boerkoel C. F., and Lupski J. R. (2001) Screening for mutations in a genetically hetero-geneous disorder: DHPLC versus DNA sequence for mutation detection in multiple genes causing Charcot-Marie-Tooth neuropathy. Genet. Med. 3(5), 335–342.
Taroni F., Pareyson D., Botti S., Sghirlanzoni A., Nemni R., and Riva D. (1999) EGR2: (Arg359Trp). Neurology 52(Suppl 2), 258–259.
Timmerman V., De Jonghe P., Ceuterick C., et al. (1999) Novel missense mutation in the early growth response 2 gene associated with Dejerine-Sottas syndrome phenotype. Neurology 52(9), 1827–1832.
Tobler A. R., Liu N., Mueller L., and Shooter E. M. (2002) Differential aggregation of the Trembler and Trembler J mutants of peripheral myelin protein 22. Proc. Natl. Acad. Sci. USA 99(1), 483–488.
Tobler A. R., Notterpek L., Naef R., Taylor V., Suter U., and Shooter E. M. (1999) Transport of Trembler-J mutant peripheral myelin protein 22 is blocked in the intermediate compartment and affects the transport of the wild-type protein by direct interaction. J. Neurosci. 19(6), 2027–2036.
Valentijn L. J., Baas F., Wolterman R. A., et al. (1992) Identical point mutations of PMP-22 in Trembler-J mouse and Charcot-Marie-Tooth disease type 1A. Nat. Genet. 2(4), 288–291.
Valentijn L. J., Baas F., Zorn I., Hensels G. W., de Visser M., and Bolhuis P. A. (1993) Alternatively sized duplication in Charcot-Marie-Tooth disease type 1A. Hum Mol. Genet. 2(12), 2143–2146.
Vallat J. M. (2003) Dominantly inherited peripheral neuropathies. J. Neuropathol. Exp. Neurol. 62(7), 699–714.
Vallat J. M., Sindou P., Preux P. M., et al. (1996) Ultrastructural PMP22 expression in inherited demyelinating neuropathies. Ann. Neurol. 39(6), 813–817.
Vance J. M., Nicholson G. A., Yamaoka L. H., et al. (1989) Linkage of Charcot-Marie-Tooth neuropathy type 1A to chromosome 17. Exp. Neurol. 104(2), 186–189.
Venken K., Di Maria E., Bellone E., et al. (2002) Search for mutations in the EGR2 corepressor proteins, NAB1 and NAB2, in human peripheral neuropathies. Neurogenetics 4(1), 37–41.
Warner L. and Lupski J. (2005) Disorders Related to EGR 2 Mutations, in Peripheral Neuropathy 2005, 4th ed., Dyck, P. J. and Thomas P. K. eds. Elsevier Saunders, Philadelphia, pp. 1707–1715.
Warner L. E., Mancias P., Butler I. J., et al. (1998) Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat. Genet. 18(4), 382–384.
Warner L. E., Svaren J., Milbrandt J., and Lupski J. R. (1999) Functional consequences of mutations in the early growth response 2 gene (EGR2) correlate with severity of human myelinopathies. Hum. Mol. Genet. 8(7), 1245–1251.
Xu W., Manichella D., Jiang H., et al. (2000) Absence of P0 leads to the dysregulation of myelin gene expression and myelin morphogenesis. J. Neurosci. Res. 60(6), 714–724.
Yamamoto M., Yoshihara T., Hattori N., and Sobue G. (2004) Glu528del in NEFL is a polymorphic variant rather than a disease-causing mutation for Charcot-Marie-Tooth disease in Japan. Neurogenetics 5(1), 75–77.
Yoshihara T., Kanda F., Yamamoto M., et al. (2001) Anovel missense mutation in the early growth response 2 gene associated with late-onset Charcot—Marie— Tooth disease type 1. J. Neurol. Sci. 184(2), 149–153.
Yoshihara T., Yamamoto M., Hattori N., et al. (2002) Identification of novel sequence variants in the neurofilament-light gene in a Japanese population: analysis of Charcot-Marie-Tooth disease patients and normal individuals. J. Peripher. Nerv. Syst. 7(4), 221–224.
Yoshikawa H., Nishimura T., Nakatsuji Y., et al. (1994) Elevated expression of messenger RNA for peripheral myelin protein 22 in biopsied peripheral nerves of patients with Charcot-Marie-Tooth disease type 1A. Ann. Neurol. 35(4), 445–450.
Young P., Stogbauer F., Eller B., et al. (2000) PMP22 Thr118Met is not a clinically relevant CMT1 marker. J. Neurol. 247(9), 696–700.
Zephir H., Stojkovic T., Latour P., Hurtevent J. F., Blankaert F., and Vermersch P. (2005) A family with a novel frameshift mutation in the PMP22 gene (c.433_434insC) causing a phenotype of hereditary neuropathy with liability to pressure palsies. Neuromuscul. Disord. 15(7), 493–497.
Zhu Q., Couillard-Despres S., and Julien J. P. (1997) Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp. Neurol. 148(1), 299–316.
Zorick T. S., Syroid D. E., Brown A., Gridley T., and Lemke G. (1999) Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development 126(7), 1397–1406.
Zuchner S., Vorgerd M., Sindern E., and Schroder J. M. (2004) The novel neurofilament light (NEFL) mutation Glu397Lys is associated with a clinically and morphologically heterogeneous type of Charcot-Marie-Tooth neuropathy. Neuromuscul. Disord. 14(2), 147–157.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houlden, H., Reilly, M.M. Molecular genetics of autosomal-dominant demyelinating Charcot-Marie-Tooth disease. Neuromol Med 8, 43–62 (2006). https://doi.org/10.1385/NMM:8:1-2:43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1385/NMM:8:1-2:43