Abstract
Simultaneous radiochemotherapy (RCT) is the treatment of choice for locally advanced head and neck cancers. In order to evaluate the toxicity and the survival rates, we investigated the use of a very aggressive combination protocol that included cisplatinum and paclitaxel combined with hyperfractionated-accelerated radiotherapy. The final results of the phase II study are listed below.
For the phase II trial 32 patients (29 male, 3 female) with histologically diagnosed locally advanced non-metastatic squamous cell carcinoma of the head and neck in stage III/IV were treated from 1999 to 2003. Radiotherapy was administered as hyperfractionated-accelerated to a total dose of 70.6 Gy. The chemotherapy regime included administering cisplatinum on d 1–5 and on d 29–33 at doses of 20 mg/m2 and during the entire course of treatment paclitaxel was administered twice a week at doses of 25 mg/m2.
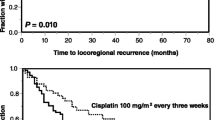
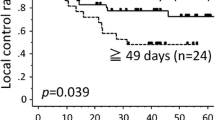
The 5-yr overall and disease-free survival rates were 48% and 43%. Twenty-two (69%) patients reached a clinically complete response and 8 (25%) a partial response (for two of the patients the response rate is not known). Two (6%) patients died during the treatment. Seven (22%) patients developed local recurrences and six of these patients have in the meantime died. With regards to the four (12%) patients who developed distant metastases, three of them have in the meantime died and two (6%) patients have died as a result of secondary malignancies.
Seven out of 25 (28%) patients developed grade 3 erythema and 22 out of 31 (71%) patients developed grade 3 mucositis. No cases of grade 4 mucositis were observed; however, one patient out of 25 (4%) was classified with grade 4 dermatitis. One out of 24 (4%) patients developed grade 2 liver toxicity and 1 out of 22 patients (5%) developed grade 3 thrombopenia. Seven out of 25 patients (28%) developed a grade 3 leukopenia, and 2 out of 25 patients (8%) experienced a grade 4 eutropenic infection. Dysphagia was a significant late toxicity. Out of 24 patients, 4 (17%) developed a grade 3 dysphagia and 1 (4%) patient developed a grade 3 xerostomia. An osteoradionecrosis was seen in 2 out of 24 (8%) patients.
Similar content being viewed by others
References
Adelstein DJ, et al. Mature results of a phase III randomized trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer 2000; 88: 876–883.
Brizel DM, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Eng J Med 1998; 338: 1798–1804.
Dobrowsky W, Naude J. Continuous hyperfractionated accelerated radiotherapy with/without mitomycin C in head and neck cancers. Radiother Oncol 2000: 57: 119–124.
Fu KK, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000; 48; 7–16.
Budach V. Bedeufung der lokalen Tumorkontrolle für das Gesamtüberleben in der Onkologie. Der Onkologe 2000; 3: 219–231.
Staar, S. et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—results of multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 50: 1161–1171.
Pignon JP, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000; 355: 949–955.
Calais G, et al. Raidomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999; 91: 2081–2086.
Wendt TG, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol 1998; 16: 1318–1324.
Liebmann JE, et al. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer 1993; 68: 1104–1109.
Liebmann JE, Fisher J, Teague D, Cook JA. Sequence dependence of paclitaxel (Taxol) combined with cisplatin or alkylators in human cancer cells. Oncol Res 1994; 6: 25–31.
Wenz F, et al. Radiochemotherapy with paclitaxel: synchronization effects and the role of p53. Strahlenther Onkol 1999; 175: 2–6.
Becker A, et al. Twice-a-week paclitaxel (PAC) and two courses cisplatin simultaneous with hyperfractionated accelerated radiotherapy for advanced squamous cell carcinoma of the head and neck (SCCHN)—phase I study. ASCO 2000 Abstract No. 1683, Category: Head and Neck Cancer.
Kuhnt T, et al. Aggressive simultaneous radiochemotherapy with cisplatin and paclitaxel in combination with accelerated hyperfractionated radiotherapy in locally advanced head and neck tumors. Results of a phase I–II trial. Strahlenther Onkol 2003; 179: 673–681.
Chougule PB, et al. Chemoradiotherapy for advanced inoperable head and neck cancer: a phase II study. Semin Radiat Oncol 1999; 9: 58–63.
Lavertu P, et al. Aggressive concurrent chemoradiotherapy for squamous cell head and neck cancer: an 8-year single-institution experience. Arch Otolaryngol Head Neck Surg 1999; 125: 142–148.
Haffty BG, et al. Chemotherapy as an adjunct to radiation in the tretment of squamous cell carcinoma of the head and neck: results of the Yale Mitomycin Randomized Trials. J Clin Oncol 1997; 15: 268–276.
Zakotnik B, et al. Concomitant radiotherapy with mitomycin C and bleomycin compared with radiotherapy alone in inoperable head and neck cancer: final report. Int J Radiat Oncol Biol Phys 1998; 41: 112.
Brockstein B, et al. A phase I–II study of concomitant chemoradiotherapy with paclitaxel (one-hour infusion), 5-fluorouracil and hydroxyurea with granulocyte colony stimulating factor support for patients with poor prognosis head and neck cancer. Ann Oncology 2000; 11: 721–728.
Forastiere AA, et al. Phase III comparison of high-dose paclitaxel + cisplain + granulocyte colony-stimulating factor versus low-dose paclitaxel + cisplatin in advanced head and neck cancer: Eastern Cooperative Oncology Group Study E1393. J Clin Oncol 2001; 19: 1088–1095.
Pradier O, et al. Effects of paclitaxel in combination with radiation on human head and neck cancer cells (ZMK-1), cervical squamous cell carcinoma (CaSki), and breast adenocarcinoma cells (MCF-7). J Cancer Res Clin Oncol 1999; 125: 20–27.
Hainsworth JD, et al. Induction paclitaxel, carboplatin, and infusional 5-FU followed by concurrent radiation therapy and weekly paclitaxel/carboplatin in the treatment of locally advanced head and neck cancer: a phase II trial of the Minnie Pearl Cancer Research Network. Cancer J 2002; 8: 311–321.
Hitt R, et al. Phase II trial of dose-dense paclitaxel, cisplatin, 5-fluorouracil, and leucovorin with filgrastim support in patients with squamous cell carcinoma of the head and neck. Cancer 2004; 101: 768–775.
Pfreundner L, et al. Induction chemotherapy with paclitaxel and cisplatin and CT-based 3D radiotherapy in patients with advanced laryngeal and hypopharyngeal carcinomas—a possibility for organ preservation. Radiother Oncol 2003; 68: 163–170.
Wang HM, et al. Biweekly paclitaxel, cisplatin, tegafur, and leucovorin as neoadjuvant chemotherapy for unresectable squamous cell carcinoma of the head and neck. Cancer 2004; 101: 1818–1823.
Hoffmann W, et al. Paclitaxel in simultaneous radiochemotherapy of head and neck cancer: preclinical and clinical results. Semin Oncol 1997; 24: 72–77.
Milano MT, et al. Phase I study of concomitant chemoradiotherapy with paclitaxel, fluorouracil, gemcitabine, and twice-daily radiation in patients with poor-prognosis cancer of the head and neck. Clin Cancer Res 2004; 10: 4922–4932.
Tishler RB, et al. An initial experience using concurrent paclitaxel and radiation in the treatment of head and neck malignancies. Int J Radiat Oncol Biol Phys 1999; 43: 1001–1008.
Abitbol A, et al. Phase II study of tolerance and efficacy of hyperfractionated radiotherapy and 5-fluorouracil, cisplatin, and paclitaxel (Taxol) in stage III and IV inoperable and/or unresectable head-and-neck squamous cell carcinoma: A-2 protocol. Int J Radiat Oncol Biol Phys 2002: 53: 942–947.
Hoffmann W, et al. Radiotherapy and concomitant weekly 1-hour infusion of paclitaxel in the tretment of head and neck cancer—results from a phase I trial. Int J Radiat Oncol Biol Phys 1997; 38: 691–696.
Machtay M, et al. A phase I trial of 96-hour paclitaxel infusion plus accelerated radiotherapy of unrespectable head and neck cancer. Int J Radiat Oncol Biol Phys 1999; 44: 311–315.
Rosen FR, et al. Multicenter randomized Phase II study of paclitaxel (1-hour infusion), fluorouracil, hydroxyurea, and concomitant twice daily radiation with or without erythro-poietin for advanced head and neck cancer. Clin Cancer Res 2003; 9: 1689–1697.
Rosenthal DI, et al. Phase I study of paclitaxel given by seven-week continuous infusion concurrent with radiation therapy for locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol 2001; 19: 1363–1373.
Steinberg L, et al. A phase I trial of radiotherapy and simultaneous 24-hour paclitaxel in patients with locally advanced head and neck squamous cell carcinoma. Semin Oncol 1997; 24: 19–56.
Suntharalingam M, et al. The use of carboplatin and paclitaxel with daily radiotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 2000; 47: 49–56.
Sunwoo JB, et al. Concurrent paclitaxel and radiation in the treatment of locally advanced head and neck cancer. J Clin Oncol 2001; 19: 800–811.
Lovey, J, et al. Radiotherapy and concurrent low-dose paclitaxel in locally advanced head and neck cancer. Radiother Oncol 2003; 68: 171–174.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuhnt, T., Becker, A., Bloching, M. et al. Phase II trial of a simultaneous radiochemotherapy with cisplatinum and paclitaxel in combination with hyperfractionated-accelerated radiotherapy in locally advanced head and neck tumors. Med Oncol 23, 325–333 (2006). https://doi.org/10.1385/MO:23:3:325
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MO:23:3:325