Abstract
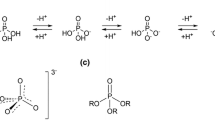
This review discusses the effects the secondary structure of an RNA molecule has on the inherent reactivity of its phosphodiester bonds, and on the catalytic activity of metal ion-based cleaving agents. The basic principles of the intramolecular transesterification of RNA phosphodiester bonds, particularly cleavage, are first briefly described. Studies of the structural effects on the cleavage, in the absence and in the presence of metal ion catalysts, are then reviewed, and the sources of the reactivity differences observed in different structures are discussed.
Similar content being viewed by others
References
Oivanen, M., Kuusela, S., and Lönnberg, H. (1998) Kinetics and mechanisms for the cleavage and isomerization of the phosphodiester bonds of RNA by Brønsted acids and bases. Chem. Rev. 98, 961–990.
Perreault, P. M. and Anslyn, E. V. (1997) Unifying the current data on the mechanism of cleavage-transesterification of RNA. Angew. Chem. Int. Ed. Engl. 36, 432–450.
Trawick, B. N., Daniher, A., and Bashkin, J. K. (1998) Inorganic mimics of ribonucleases and ribozymes: from random cleavage to sequence-specific chemistry to catalytic antisense drugs. Chem. Rev. 98, 939–960.
Zhou, D.-M. and Taira, K. (1998) The hydrolysis of RNA: From theoretical calculations to the hammerhead ribozyme-mediated cleavage of RNA. Chem. Rev. 98, 991–1026.
Cech, T., Zaugh, A. J., and Grabowski, P. J. (1981) In vitro splicing of the ribosomal RNA precursor of tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell 29, 487–496.
Guerrier-Takada, C., Gardiener, K., Marsh, T., Pace N. and Altman, S. (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35, 849–857.
Pyle, A. M. (1996) Role of metal ions in ribozymes. Metal Ions Biol. Syst. 32, 479–520.
Bashkin, J. K., Sampath, U. and Frolova, E. (1995) Ribozyme mimics as catalytic antisense reagents. Appl. Biochem. Biotech. 54, 43–56.
Vlasov, V. V., S’ilnikov, V. N. and Zenkova, M. A. (1998) Chemical ribonucleases. Mol. Biol. 32, 50–57.
Komiyama, M. (1995) Sequence-specific and hydrolytic scission of DNA and RNA by lanthanide complex-oligoDNA hybrids. J. Biochem. 118, 665–670.
De Mesmaeker, A., Häner, R., Martin, P. and Moser, H. (1995) Antisense oligonucleotides. Acc. Chem. Res. 28, 366–374.
Häner, R., Hall, J., Pfutzer, A. and Husken, D. (1998) Development of artificial ribonucleases. Pure Appl. Chem. 70, 111–116.
Sigman, D. S., Bruice, T. W., Mazumder, A. and Sutton, C. L. (1993) Targeted chemical nucleases. Acc. Chem. Res. 26, 98–104.
Duarte, V., Sixou, S., Favre, G., Pratviel, G. and Meunier, B. (1997) Oxidative damage on RNA mediated by cationic metalloporphyrin antisense oligonucleotide conjugates. J. Chem. Soc. Dalton Trans. 21, 4113–4118.
Bashkin, J. K. and Jenkins, L. A. (1994) The role of metal ions in the hydrolytic cleavage of DNA and RNA. Comments Inorg. Chem. 16, 77–93.
Kuusela, S. and Lönnberg, H. (1996) Effect of metal ions on the hydrolytic reactions of nucleosides and their phosphoesters. Metal Ions Biol. Syst. 32, 272–300.
Kuusela, S. and Lönnberg, H. (1997) Metal ion dependent hydrolysis of RNA. Curr. Topics Sol. Chem. 2, 29–47.
Morrow, J. R. (1996) Hydrolytic cleavage of RNA catalysed by metal ion complexes. Metal Ions Biol. Syst. 33, 561–592.
Blaskó, A. and Bruice, T. C. (1999) Recent studies of nucleophilic, general-acid, and metal ion catalysis of phosphate diester hydrolysis. Acc. Chem. Res. 32, 475–484.
Williams, N. H., Takasaki, B., Wall, M. and Chin, J. (1999) Structure and nuclease activity of simple dinuclear metal complexes: quantitative dissection of the role of metal ions. Acc. Chem. Res. 32, 485–493.
Daniher, A. T. and Bashkin, J. K. (1998) Precise control of RNA cleavage by ribozyme mimics. Chem. Commun. 1077–1078.
Bashkin, J. K., Frolova, E. and Sampath, U. (1994) Sequence-specific cleavage of HIV mRNA by a ribozyme mimic. J. Am. Chem. Soc. 116, 5981–5982.
Magda, D., Crofts, S., Lin, A., Miles, D., Wright, M. and Sessler, J. L. (1997) Synthesis and kinetic properties of ribozyme analogues prepared using phosphoramidate derivatives of dysprosium(III) texapyrin. J. Am. Chem. Soc. 119, 2293–2294.
Matsuda, S., Ishikubo, A., Kuzuya, A., Yashiro, M. and Komiyama, M. (1998) Conjugates of a dinuclear zinc(II) complex and DNA oligomers as novel sequence-selective artificial ribonucleases. Angew. Chem. Int. Edit. Engl. 37, 3284–3286.
Hall, J., Husken, D., Pieles, U., Moser, H. E. and Häner, R. (1994) Efficient sequence-specific cleavage of RNA using novel europium complexes conjugated to oligonucleotides. Chem. Biol. 1, 185–190.
Inoue, H., Furukawa, T., Shimizu, T., Tamura, T., Matsui, M. and Ohtsuka, E. (1999) Efficient site-specific cleavage of RNA using a terpyridine-copper(II) complex joined to a 2′-O-methyloligonucleotide by a non-flexible linker. Chem. Commun. 45–46.
Hovinen, J., Guzaev, A., Azhayeva, E., Azhayev, A. and Lönnberg, H. (1995) Imidazole tethered oligodeoxyribonucleotides: synthesis and RNA cleaving activity. J. Org. Chem. 60, 2205–2209.
Nicholson, A. W. (1996) Structure, reactivity and biology of double-stranded RNA. Prog. Nucleic Acid Res. Mol. Biol., 52, 1–56.
Kierzek, R. (1992) Nonenzymatic hydrolysis of oligoribonucleotides. Nucleic Acids Res. 20, 5079–5084.
Kolasa, K. A., Morrow, J. R., and Sharma, A. P. (1993) Trivalent lanthanide ions do not cleave RNA in DNA-RNA hybrids. Inorg. Chem. 32, 3983–3984.
Hüsken, D., Goodall, G., Blommers, M. J. J., Jahnke, W., Hall, J., Häner, R. and Moser, H. E. (1996) Creating RNA bulges: cleavage of RNA in RNA/DNA duplexes by metal ion catalysis. Biochemistry 35, 16591–16600.
Zagorowska, I., Kuusela, S. and Lönnberg, H. (1998) Metal ion-dependent hydrolysis of RNA phosphodiester bonds within hairpin loops. A comparative kinetic study on chimeric ribo/2′-O-methylribo oligoribonucleotides. Nucleic Acids Res. 26, 3392–3396.
Cuchillo, C. M., Pares, X., Guasch, A., Barman, T., Travers, F. and Nogues, M. V. (1993) The role of 2′,3′-cyclic phosphodiesters in the bovine pancreatic ribonuclease A catalysed cleavage of RNA: Intermediates or products. FEBS Lett. 333, 207–210.
Loll, P. and Lattman, E. E. (1989) The crystal structure of the ternary complex of staphylococcal nuclease, calcium, and the inhibitor pdTp. Proteins Struct. Funct. Genet. 5, 183–201.
Zagórowska, I., Mikkola, S. and Lönnberg, H. (1999) Hydrolysis of phosphodiester bonds within hairpin loops in buffer solutions: the effect of secondary structure on the inherent reactivity of RNA phosphodiester bonds. Helv. Chim. Acta 82, 2105–2111.
Brown, R. S., Dewan, J. C. and Klug, A. (1985) Crystallographic and biochemical investigation of the lead(II)-catalyzed hydrolysis of yeast phenylalanine tRNA. Biochemistry 24, 4785–4801.
Sundaralingam, M., Rubin, J. R. and Cannon, J. C. (1984) Nonenzymatic hydrolysis of RNA: Pb(II)-catalyzed site specific hydrolysis of tRNA. The role of the tertiary folding of the polynucleotide chain. Int J. Quant. Chem. 11, 355–366.
Wrzesinski, J., Michalowski, D., Ciesiolka, J. and Krzyzosiak, W. J. (1995) Specific RNA cleavages induced by manganese ions. FEBS Lett. 374, 62–68.
Eckstein, F. and Lilley, D. M. (1996) Catalytic RNA, Springer, Berlin.
Hall, J., Hüsken, D. and Häner, R. (1996) Towards artificial ribonucleases: the sequence-specific cleavage of RNA in a duplex. Nucleic Acids Res. 24, 3522–3526.
Westheimer, F. H. (1968) Pseudo-rotation in the hydrolysis of phosphate esters. Acc. Chem. Res. 1, 70–78.
Järvinen, P., Oivanen, M. and Lönnberg, H. (1991) Interconversion and phosphoester hydrolysis of 2′,5′- and 3′,5′-dinucleoside monophosphates: Kinetics and mechanism. J. Org. Chem. 56, 5396–5401.
Kosonen, M., Oivanen, M. and Lönnberg, H. (1994) Hydrolysis and interconversion of the dimethyl esters of 5′-O-methyluridine 2′- and 3′-monophosphates: kinetics and mechanism, J. Org. Chem. 59, 3704–3708.
Kosonen, M. and Lonnberg, H. (1995) General and specific acid/base catalysis of the hydrolysis and interconversion of ribonucleoside 2′- and 3′-phosphotriesters: kinetics and mechanisms of the reactions of 5′-O-pivaloyl 2′- and 3′-dimethylphosphates. J. Chem. Soc. Perkin Trans. 2, 1203–1209.
Kosonen, M., Hakala, K. and Lönnberg, H. (1998) Hydrolysis and intramolecular transesterification of ribonucleoside 3′-phosphotriesters: the effect of alkyl groups on the general and specific acid-base-catalyzed reactions of 5′-O-pivaloyluridin-3′-yl dialkyl phosphates. J. Chem. Soc. Perkin Trans. 2, 663–670.
Kosonen, M., Yousefi-Salakdeh, E., Strömberg, R., and Lönnberg, H. (1998) pH- and buffer-independent cleavage and mutual isomerization of uridine 2′-and 3′-alkyl phosphodiesters: implications for the buffer catalyzed cleavage of RNA. J. Chem. Soc. Perkin Trans. 2, 1589–1595.
Oivanen, M., Schnell, R., Pfleiderer, W. and Lönnberg, H. (1991) Interconversion and hydrolysis of monomethyl and monoisopropyl esters of adenosine 2′-and 3′-monophosphates: kinetics and mechanism. J. Org. Chem. 56, 3625–3628.
Li, Y. and Breaker, R. R. (1999) Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J. Am. Chem. Soc. 121, 5364–5372.
Kosonen, M., Yousefi-Salakdeh, E., Strömberg, R. and Lönnberg, H. (1997) Mutual isomerisation of uridine 2′-and 3′-alkylphosphates and cleavage to 2′,3′-cyclic phosphate: the effect of the alkyl group on the hydronium and hydroxide-ion-catalysed cleavage. J. Chem. Soc. Perkin Trans. 2, 2661–2666.
Dejaegere, A., Liang, X. and Karplus, M. (1994) Phosphate ester hydrolysis: Calculation of gasphase reaction paths and solvation effects. J. Chem. Soc. Faraday Trans. 90, 1763–1770.
Anslyn, E. and Breslow, R. (1989) On the mechanism of catalysis by ribonuclease; cleavage and isomerizarion of the dinucleotide UpU catalysed by imidazole buffers. J. Am. Chem. Soc. 111, 4473–4482.
Breslow, R., Dong, S. D., Webb, Y. and Xu, R. (1996) Further studies on the buffer-catalyzed cleavage and isomerization of uridylyluridine. Medium and ionic strentgh effects on catalysis by morpholine, imidazole and acetate buffers help clarify the mechanisms involved and their relationship to the mechanism used by the enzyme ribonuclease and by a ribonuclease mimic. J. Am. Chem. Soc. 118, 6588–6600.
Beckmann, C., Kirby, A. J., Kuusela, S. and Tickle, D. C. (1998) Mechanism of catalysis by imidazole buffers of the hydrolysis and isomerisation of RNA models. J. Chem. Soc. Perkin Trans. 2., 573–581.
Menger, F. M. (1991) The negative rate constants of Breslow and Huang. J. Org. Chem. 56, 6251–6252.
Haim, A. (1992) Imidazole buffer-catalyzed cleavage and isomerization reactions of dinucleotides: the proposed mechanism is incompatible with the kinetic measurements. J. Am. Chem. Soc. 114, 8384–8388.
Perrin, C. L. (1995) On the mechanism of buffer-catalysed hydrolysis of RNA models. J. Org. Chem. 60, 1239–1243.
Kuusela, S. and Lönnberg, H. (1992) Metal ion promoted hydrolysis of uridine 2′,3′-cyclic monophosphate of uridine. J. Phys. Org. Chem. 5, 803–811.
Kuusela, S. and Lönnberg, H. (1993) Metal ions that promote the hydrolysis of nucleoside phosphoesters do not enhance intramolecular phosphate migration. J. Phys. Org. Chem. 6, 347–356.
Kuusela, S., Rantanen, M. and Lönnberg, H. (1995) Metal-ion-promoted hydrolysis of uridylyl(3′,5′)uridine: internal vs. external genaral base catalysis. J. Chem. Soc. Perkin Trans. 2, 2269–2273.
Mikkola, S., Stenman, E., Nurmi, K., Yousefi-Salakdeh, E., Strömberg, R. and Lönnberg H. (1999) The mechanism of the metal ion promoted cleavage of RNA phosphodiester bonds involves a general acid catalysis by the metal aquo ion on the departure of the leaving group. J. Chem. Soc. Perkin Trans. 2, 1619–1625.
Komiyama, M., Matsumoto, Y., Takahashi, H., Shiiba, T., Tsuzuki, H., Yajima, H., Yashiro, M. and Sumaoka, J. (1998) RNA hydrolysis by cobalt(III) complexes. J. Chem. Soc. Perkin Trans. 2, 691–695.
Koike, T. and Kimura, E. (1991) Roles of zinc(II) ion in phosphatases. A model study with zinc(II)-macrocyclic polyamine complexes. J. Am. Chem. Soc. 113, 8935–8941.
Oivanen, M., Mikhailov, S. N., Florentiev, V. L., Vihanto, P., and Lönnberg, H. (1993) Interconversion and hydrolysis of 1-[(2′S)-2′,3′-dihydroxypropyl]cytosine analogues of isomeric dinucleoside monophosphates, 2′,5′-CpA and 3′,5′-ApA. Acta Chem. Scand. 47, 622–625.
Holy, A. and Ivanova, G. S. (1974) Aliphatic analogs of nucleotides. Synthesis and affinity towards nucleases. Nucleic Acids Res. 1, 19–34.
Avramova, Z. V. and Dudkin, S. M. (1978) Role of the substrate ribose residues in reactions catalyzed by ribonucleases. Mol. Biol. (USSR) 12, 914–921.
Witzel, H. (1960) Einfluss der nucleotidbasen auf die nicht-enzymatische spaltung der ribonucleinsäure-diester bindungen. Liebigs Ann. Chem. 635, 182–191.
Abrash, H. I., Cheung, C.-C. S. and Davis, J. C. (1967) The nonenzymic hydrolysis of nucleoside 2′,3′-phosphates. Biochemistry 6, 1298–1303.
Oivanen, M. and Lönnberg, H. (1991) Hydrolysis and intramolecular transesterification of ribonucleoside phosphoesters. Trends Org. Chem. 2, 183–198.
Kuusela, S. and Lönnberg, H. (1994) Hydrolysis and isomerization of the internucleosidic phosphodiester bonds of polyuridylic acid: kinetics and mechanism. J. Chem. Soc. Perkin Trans. 2, 2109–2113.
Butzow, J. J. and Eichhorn, G. L. (1971) Interaction of metal ions with nucleic acids and related compounds. XVII. On the mechanism of degradation of polyribonucleotides and oligoribonucleotides by zinc(II) ions. Biochemistry 11, 2019–2027.
Kuusela, S., Azhayev, A., Guzaev, A. and Lönnberg, H. (1995) The effect of the 3′-terminal monophosphate group on the metal-ion-promoted hydrolysis of the phosphodiester bonds of short oligonucleotides. J. Chem. Soc. Perkin Trans. 2, 1197–1202.
Kuusela, S. and Lönnberg, H. (1998) Catalytically significant macrochelate formation in Zn2+ promoted hydrolysis of oligoribonucleotides: model studies with chimeric phosphodiester/methylphosphonate oligomers. Nucleosides Nucleotides 17, 2417–2427.
Kuusela, S. and Lönnberg, H. (1994) Metal-ion-promoted hydrolysis of polyuridylic acid. J. Chem. Soc. Perkin Trans 2, 2301–2306.
Kuusela, S. and Lönnberg, H. (1994) Zn2+ promoted hydrolysis of 3′,5′-dinucleoside monophosphates and polyribonucleotides. The effect of nearest neighbours on the cleavage of phosphodiester bonds. Nucleosides Nucleotides 15, 1669–1678.
Ikenaga, H. and Inoue, Y. (1974) Metal(II) ion catalyzed transphosphorylation of four homodinucleotides and five pairs of dinucleotide sequence isomers. Biochemistry 13, 577–582.
Kuusela, S., Guzaev, A. and Lönnberg, H. (1996) Acceleration of the Zn2+ promoted phosphodiester hydrolysis of oligonucleotides by the 3′-terminal monophosphate group: intrastrand participation over several nucleoside units. J. Chem. Soc. Perkin Trans. 2, 1895–1899.
Breslow, R. and Huang, D.-L. (1991) Effects of metal ions, including Mg2+ and lanthanides, on the cleavage of ribonucleotides and RNA model compounds. Proc. Natl. Acad. Sci. USA 88, 4080–4083.
Morrow, J. R., Buttrey, L. A. and Berback, K. A. (1992) Transesterification of a phosphate diester by divalent and trivalent metal ions. Inorg. Chem. 31, 16–20.
Saenger, W. (1984) The Structure of Nucleic Acids, Springer, Berlin.
Usher, D. A. and McHale, A. H. (1976) Hydrolytic stability of helical RNA; a selective advantage for the natural 3′,5′-bond. Proc. Natl. Acad. Sci. USA 73, 1149–1153.
Zaug, A. J. and Cech, T. R. (1986) The intervening sequence RNA of Tetrahymena is an enzyme. Science 231, 470–475.
Vlassov, V. V., Zuber, G., Felden, B., Behr, J.-P., and Giege, R. (1995) Cleavage of tRNA with imidazole constructs: a new approach for probing RNA structure. Nucleic Acids Res. 23, 3161–3167.
Reynolds, M. A., Beck, T. A., Say, P. B., Schwartz, D. A., Dwyer, B. P., Daily, W. J., Vaghefi, M. M., Metzler, M. D., Klem, R. E., and Arnold, L. J., Jr. (1996) Antisense oligonucleotides containing an internal non-nucleotide-based linker promote site-specific cleavage of RNA. Nucleic Acids Res. 24, 760–765.
Hayashi, N., Takeda, N., Shiiba, T., Yashiro, M., Watanabe, K. and Komiyama, M. (1993) Site-selective hydrolysis of tRNA by lanthanide metal complexes. Inorg. Chem. 32, 5899–5900.
Soukup, G. A. and Breaker, R. R. (1999) Relationship between internucleosidic linkage geometry and the stability of RNA. RNA 5, 1308–1325.
Laing, L. G. and Hall, K. B. (1996) A model of the iron responsive element RNA hairpin loop structure determined from NMR and thermodynamic data. Biochemistry 35, 13,586–13,596.
Molinaro, M. and Tinoco, I., Jr. (1995) Use of ultra stable UNCG tetraloop hairpins to fold RNA structures: thermodynamics and spectroscopic applications. Nucleic Acids Res. 23, 3056–3063.
Portmann, S., Grimm, S., Workman, C., Usman, N., and Egli, M. (1996) Crystal structures of an A-form duplex with single-adenosine bulges and a conformational basis for site-specific RNA self-cleavage. Chem. Biol. 3, 173–184.
Endo, M., Hirata, K., Ihara, T., Sueda, S. S., Takagi, M., and Komiyama, M. (1996) RNA hydrolysis by the cooperation of the carboxylate ion and ammonium ion. J. Am. Chem. Soc. 118, 5478–5479.
Podymigonin, M. A., Vlassov, V. V., and Giege, R. (1993) Synthetic RNA-cleaving molecules mimicking ribonuclease A active center. Design and cleavage of tRNA transcripts. Nucleic Acids Res. 21, 5950–5956.
Keck, M. V. and Hecht, S. M. (1995), Sequence-specific hydrolysis of yeast tRNAPhe mediated by metal-free bleomycin. Biochemistry 34, 12,029–12,037.
Sreedhara, A., Patwardhan, A., and Cowan, A. A. (1999) Novel reagents for targeted cleavage of RNA sequences: towards a new family of inorganic pharmaceuticals. Chem. Commun., 1147–1148.
Hegg, E. L., Deal, K. A., Kiessling, L. L., and Burstyn, J. N. (1997) Hydrolysis of double-stranded and single-stranded RNA in hairpin structures by the copper(II) macrocycle Cu([9]aneN3)Cl2 Inorg. Chem. 36, 1715–1718.
Kierzek, R. (1992). Hydrolysis of oligoribonucleotides: Influence of sequence and length. Nucleic Acids Res. 20, 5073–5077.
Hosaka, H., Sakabe, I., Sakamoto, K., Yokoyama, S., and Takaku, H. (1994) Sequence-specific cleavage of oligoribonucleotide capable of forming a stem and loop structure. J. Biol. Chem. 269, 20,090–20,094.
Ciesiolka, J., Lorenz, S., and Erdmann, V. A. (1992) Different conformational forms of Escherichia coli and rat liver 5SrRNA revealed by Pb(II)-induced hydrolysis. Eur. J. Biochem. 204, 583–589.
Lane, B. G. and Butler, G. C. (1959) The exceptional resistance of certain oligoribonucleotides to alkaline degradation. Biochim. Biophys. Acta 33, 281–283.
Smith, K. C. and Allen, F. W. (1953) The liberation of polynucleotides by the alkaline hydrolysis of ribonucleic acid from yeast. J. Am. Chem. Soc. 75, 2131–2133.
Norberg, J. and Nilsson, L. (1995) Stacking free energy profiles for all 16 natural ribodinucleoside monophosphates in aqueous solution. J. Am. Chem. Soc. 117, 10,832–10,840.
Ciesiolka, J., Michalowski, D., Wrzesinski, J., Krajewski, J., and Krzyzosiak, W. J. (1998) Patterns of cleavage induced by lead ions in defined RNA secondary structure motifs. J. Mol. Biol. 275, 211–220.
Ciesiolka, J., Gorski, Y., and Yarus, M. (1995) Selection of an RNA domain that binds Zn2+. RNA 1, 538–550.
Ciesiolka, J. and Krzyzosiak, W. J. (1996) Structural analysis of two plant 5S rRNA species and fragments thereof by lead-induced hydrolysis. Biochem. Mol. Biol. Int. 39, 319–328.
Ciesiolka, J., Lorenz, S., and Erdmann, V. A. (1992) Structural analysis of three prokaryotic 5S rRNA species and selected 5S rRNA-ribosomal-protein complexes by means of Pb(II)-induced hydrolysis. Eur. J. Biochem. 204, 575–581.
Cheong, Ch., Varani, G., and Tinoco, I., Jr. (1990) Solution structure of an unusually stable RNA hairpin, 5′-GGAC(UUCG)GUCC. Nature 346, 680–682.
Heus, H. A. and Pardi, A. (1991) Structural features that give rise to the unusual stability of RNA hairpins containing GNRA loops. Science 253, 191–194.
Farkas, W. R. (1968) Depolymerisation of ribonucleic acid by plumbous ion. Biochim. Biophys. Acta 155, 401–409.
Brown, R. S., Hingerty, B. E., Dewan, J. C., and Klug, A. (1983) Pb(II)-catalysed cleavage of the sugar-phosphate backbone of yeast tRNAPhe—implications for lead toxicity and self-splicing RNA. Nature 303, 543–546.
Michalowski, D., Wrzesinski, J., Ciesiolka, J., and Krzyzosiak, W. J. (1996) Effect of modified nucleotides on structure of yeast thRNAPhe. Comparative studies by metal ion-induced hydrolysis and nuclease mapping. Biochimie 78, 131–138.
Michalowski, D., Wrzesinski, J., and Krzyzosiak, W. J. (1996) Cleavages induced by different metal ions in Yeast tRNAPhe U59C60 mutants. Biochemistry 35, 10,727–10,734.
Behlen, L. S., Sampson, J. R., DiRenzo, A. B., and Uhlenbeck, O. C. (1990) Lead-catalysed cleavage of yeast tRNAPhe mutants. Biochemistry 29, 2515–2523.
Rordorf, B. T. and Kearns, D. R. (1976) Effect of europium(III) on the thermal denaturation and cleavage of transfer ribonucleic acids. Biopolymers 15, 1491–1504.
Ciesiolka, J., Marciniec, T., and Krzyzosiak, W. J. (1989) Probing the environment of lanthanide binding sites in yeast tRNAPhe by specific metal-ion-promoted cleavages. Eur. J. Biochem. 182, 445–450.
Pan, T. and Uhlenbeck, O. C. (1992) In vitro selection of RNA’s that undergo autolytic cleavage in the presence of Pb2+. Biochemistry 31, 3887–3895.
Pan, T. and Uhlenbeck, O. C. (1992) A small metalloribozyme with a two-step mechanism. Nature 358, 560–563.
Ohmichi, T. and Sugimoto, N. (1997) Role of Nd3+ and Pb2+ on the RNA cleavage reaction by a small ribozyme. Biochemistry 36, 3514–3521.
Pan, T., Dichtl, B., and Uhlenbeck, O. C. (1994) Properties of an in vitro selected Pb2+ cleavage motif. Biochemistry 33, 9561–9565.
Chartrand, P., Usman, N., and Cedergren, R. (1997) Effect of structural modifications on the activity of the leadzyme. Biochemistry 36, 3145–3150.
Wedekind, J. E. and McKay, D. B. (1999) Crystal structure of a lead-dependent ribozyme revealing metal binding sites relevant to catalysis. Nature Struct. Biol. 6, 261–268.
Hoogstraten, C. G., Legault, P., and Pardi, A. (1998) NMR solution structure of the lead-dependent ribosyme: evidence for dynamics in RNA catalysis. J. Mol. Biol. 284, 337–350.
Legault, P., Hoogstraten, C. G., Metlitzky, E., and Pardi, A. (1998) Order, dynamics and metal-binding in the lead-dependent ribozyme. J. Mol. Biol. 284, 325–335.
Dange, V., Van Atta, R. B., and Hecht, S. M. (1990) A Mn2+ dependent ribozyme. Science 248, 585–588.
Kazakov, S. and Altman, S. (1992) A trinucleotide can promote metal ion-dependent specific cleavage of RNA. Proc. Natl. Acad. Sci. USA 89, 7939–7943.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mikkola, S., Kaukinen, U. & Lönnberg, H. The effect of secondary structure on cleavage of the phosphodiester bonds of RNA. Cell Biochem Biophys 34, 95–119 (2001). https://doi.org/10.1385/CBB:34:1:95
Issue Date:
DOI: https://doi.org/10.1385/CBB:34:1:95