Abstract
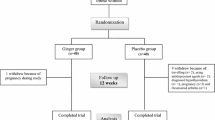
Leptin is thought to be a lipostatic signal that contributes to body weight regulation. Zinc might play an important role in appetite regulation and its administration stimulates leptin production. However, there are few reports in the literature on its role on leptin levels in the obese population. The present work asseses the effect of zinc supplementation on serum leptin levels in insulin resistance (IR). A prospective double-blind, randomized, clinical, placebo-controlled study was conducted. Fifty-six normal glucose-tolerant obese women (age: 25–45 yr, body mass index [BMI]=36.2 ±2.3 kg/m2) were randomized for treatment with 30 mg zinc daily for 4 wk. Baseline values of both groups were similar for age, BMI, caloric intake, insulin concentration, insulin resistance, and zinc concentration in diet, plasma, urine, and erythrocytes. Insulin and leptin were measured by radioimmunoassay and IR was estimated by the homeostasis model assessment (HOMA). The determinations of zinc in plasma, erythrocytes, and 24-h urine were performed by using atomic absorption spectrophotometry. After 4 wk, BMI, fasting glucose, and zinc concentration in plasma and erythrocyte did not change in either group, although zinc concentration in the urine increased from 385.9±259.3 to 470.2±241.2±μg/24 h in the group with zinc supplementation (p<0.05). Insulin did not change in the placebo group, whereas there was a significant decrease of this hormone in the supplemented group. HOMA also decreased from 5.8±2.6 to 4.3±1.7 (p<0.05) in the zinc-supplemented group but did not change in the placebo group. Leptin did not change in the placebo group. In the zinc group, leptin was 23.6±12.3 μg/L and did not change. More human data from a unique population of obese individuals with documented insulin resistance would be useful in guiding future studies on zinc supplementation (with higher doses or longer intervals) or different measures.
Similar content being viewed by others
References
G. M. Reaven and A. Laws, Insulin resistance, compensatory hyperinsulinemia, and coronary heart disease, Diabetologia 37, 948–952 (1994).
J. P. Despres, B. Lamarche, and P. Mauriege, Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 334, 952–957 (1996).
A. O. MacDougald, C. S. Hwang, H. Fan, and M. D. Lane, Regulated expression of the obese product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 92, 9034–9037 (1995).
J. Rentsch and M. Chiesi, Regulation of ob gene mRNA levels in cultured adipocytes, FEBS Lett. 379, 55–59 (1996).
J. I. Halaas, K. S. Gajiwala, and M. Maffei, Weight-reducing effects of the plasma protein encoded by the obese gene, Science 269, 543–546 (1995).
T. W. Stephens, M. Basinski, and P. K. Bristow, The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377, 530–532 (1995).
R. V. Considine, M. K. Sinha, and M. L. Heiman, Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
B. S. Hamilton, D. Paglia, A. Y. M. Kwan, and M. Deitel, Increased obese nRNA expression in omental fat cells from massively obese humans. Nature Med. 1, 953–956 (1995).
S. M. Haffner, H. Miettinen, L. Mykkanen, D. L. Rainwater, and M. Laakso, Leptin concentrations and insulin sensitivity in normoglycemic men. Int. J. Related Metab. Disord. 21, 393–399 (1997).
M. D. Chen, P. Lin, and W. Sheu, Zinc status in plasma of obese individuals during glucose administration, Biol. Trace Element Res. 60, 123–129 (1997).
G. Martino, M. G. Matera, B. Martino, C. Vacca, S. Martino, and F. Rossi, Relationship between zinc and obesity, J. Med. 24, 177–183 (1993).
L. Perrone, G. Gialanella, R. Moro, et al., Zinc, copper, and iron in obese children and adolescents. Nutr. Res. 18, 183–189 (1998).
C. S. Mantzoros, A. S. Prasad, F. W. J. Beck, et al., Zinc may regulate serum leptin concentrations in humans, J. Am. Coll. Nutr, 17, 270–275 (1998).
L. Coulston and P. Dandona, Insulin-like effects of zinc on adipocytes, Diabetes 29, 665–667 (1980).
Biodynamics, Monitor de composição corporal: biodynamics modelo 310, Biodynamics, (1995).
R. C. Whitehouse, A. S. Prasad, P. I. Rabbani, and Z. T. Cossack, Zinc in plasma, neutrophils lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry, Clin. Chem. 28, 475–480 (1982).
M. P. Rodriguez, A. Narizano, V. Demczylo, and A. Cid, A simpler method for the determination of zinc human plasma levels by flame atomic absorption spectrophotometry, Atomic Spectrosc. 10, 68–70 (1989).
O. W. Van Assendelft. The measurement of hemoglobin, in Modern Concepts in Hematology, G. Izak and S. M. Lewis (eds.), Academic, New York, pp. 4–25 (1972).
S. Kilerich, M. S. Christiansen, and J. Naestoft. Determination of zinc in serum and urine by atomic absorption spectrophotometry; relationship between serum levels of zinc and proteins in 104 normal subjects. Clin. Chim. Acta 105, 231–239 (1980).
D. R. Matthews, J. P. Hosker, A. S. Rudenski, B. A. Naylor, D. F. Treacher, and R. C. Turner, Homeostasis Model Assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
J. C. King and C. L. Keen, Zinc, in Modern Nutrition in Health and Disease, 8th ed., M. E. Shils, J. A. Olson, and M. Shike, eds., Lea and Febiger, Philadelphia, pp. 214–230 (1994).
D. L. Donaldson, C. C. Smith, and M. S. Walker, Tissue zinc and copper levels in diabetic C57BL/KsJ (ob/ob) mice fed a zinc-deficient diet: lack of evidence for specific depletion of tissue zinc stores. J. Nutr. 118, 1502–1508 (1988).
N. Begin-heick, M. Dalpe-Scott, J. Rowe, and H. M. C. Heick, Zinc supplementation attenuates secretory activity in pancreatic islets of the ob/ob mouse, Diabetes 34, 179–184 (1985).
D. N. Marreiro, M. Fisberg, and S. M. F. Cozzolino, Zinc nutritional status in obese children and adolescents. Biol. Trace Element Res. 85, 1–16 (2002).
Food and Nutrition Board, Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chormium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc, National Academy of Sciences, Washington, DC, (2001).
G. J. Brewer, V. Yuzbasiyan-gurkan, V. Johnson, R. D. Dick, and Y. Wang, Treatment of Wilson's disease with zinc: XI. Interation with other anticopper agents, J. Am. Coll. Nutr. 12, 26–30 (1993).
M. L. Kennedy and M. L. Failla, Zinc metabilsm in genetically obese (ob/ob) mice, J. Nutr. 117, 886–893 (1987).
D. N. Marreiro, M. Fisberg, and S. M. F. Cozzolino, Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents, Biol. Trace Element Res. 100, 137–150 (2004).
M. D. Chen, Y. M. Song, and P. Y. Lin, Zinc effects on hyperglycemia and hypoleptinemia in streptozotocin-induced diabetic mice. Horn. Metab. Res. 32, 107–109 (2000).
P. Sarrat, R. C. Frederich, E. M. Turner, et al., Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia, J. Exp. Med. 185, 171–175 (1997).
T. G. Kirchgessner, K. T. Uysal, S. M. Wiesbrock, M. W. Marino, and G. S. Hotamisligil Tumor necrosis factor a contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J. Clin. Invest. 100, 2777–2782 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marreiro, D.d.N., Geloneze, B., Tambascia, M.A. et al. Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol Trace Elem Res 112, 109–118 (2006). https://doi.org/10.1385/BTER:112:2:109
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:112:2:109