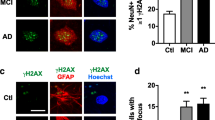
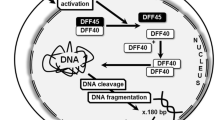
Abstract
Analysis of DNA fragmentation using Terminal deoxynucleotidyl Transferase (TdT)-mediated nick end-labeling (TUNEL) is a very sensitive technique for in situ detection of various types of DNA breaks in cells undergoing apoptosis and/ or necrosis (1–6). TUNEL technique is widely used, for instance, to study mechanisms underlying early development and morphogenesis (7–14), aging (15–24), cancer (25,5,25–35) and neurodegenerative diseases (36,20,21,36–44). TUNEL detects the DNA fragmentation, which represents the end point of DNA degradation in apoptosis but does not depict primary stimuli that caused irreversible disruption to the integrity of DNA. Oxidative stress is one of such primary stimuli and there is a great deal of research aimed at unraveling the molecular mechanisms underlying oxidative damage to DNA by so-called reactive oxygen species (ROS) and oxygen radicals. Oxidative damage has been implicated in a wide variety of neurodegenerative disorders including Alzheimer’s dementia, amyotrophic lateral sclerosis, Huntington’s disease and Parkinson’s disease (45–55). Formation of 8-hydroxy-2′-deoxyguanosine (8-OHdG) is the most common modification of DNA caused by oxidative stress. Therefore, immunohistochemical quantification of 8-OHdG would be a valuable tool in determining the extent of oxidative DNA damage caused by ROS. On the other hand, methods analyzing the oxidative damage to the DNA, are not sufficient alone either, since they do not reveal whether oxidative stress will result in apoptosis and cell death or not. Thus, it appears that limitations of each individual assay may be overcome if both techniques are combined in a single assay that is applied to the same cytological of histological specimen.
Similar content being viewed by others
References
Ansari B., Coates P. J., Greenstein B. D., and Hall P. A. (1993) In situ end-labeling detects DNA strand breaks in apoptosis and other physiological and pathological states. J. Pathol. 170, 1–8.
Allen R. T., Hunter W. J., 3rd, and Agrawal D. K. (1997) Morphological and biochemical characterization and analysis of apoptosis. J. Pharmacol. Toxicol. Methods 37, 215–228.
Heatwole V. M. (1999) TUNEL assay for apoptotic cells. Methods Mol. Biol. 115, 141–148.
Takashi E. and Ashraf M. (2000) Pathologic assessment of myocardial cell necrosis and apoptosis after ischemia and reperfusion with molecular and morphological markers. J. Mol. Cell Cardiol. 32, 209–224.
Mangili F., Cigala C., and Santambrogio G. (1999) Staining apoptosis in paraffin sections. Advantages and limits. Anal. Quant. Cytol. Histol. 21, 273–276.
van Lookeren Campagne M., Lucassen P. J., Vermeulen J. P., and Balazs R. (1995) NMDA and kainate induce internucleosomal DNA cleavage associated with both apoptotic and necrotic cell death in the neonatal rat brain. Eur. J. Neurosci. 7, 1627–1640.
Liu L. and Keefe D. L. (2000) Cytoplasm mediates both development and oxidation-induced apoptotic cell death in mouse zygotes. Biol. Reprod. 62, 1828–1834.
Marin-Teva J. L., Cuadros M. A., Calvente R., Almendros A., and Navascues J. (1999) Naturally occurring cell death and migration of microglial precursors in the quail retina during normal development. J. Comp. Neurol. 412, 255–275.
Bessert D. A. and Skoff R. P. (1999) High-resolution in situ hybridization and TUNEL staining with free-floating brain sections. J. Histochem. Cytochem. 47, 693–702.
Maciejewska B., Lipowska M., Kowianski P., Domaradzka-Pytel B., and Morys J. (1998) Postnatal development of the rat striatum—a study using in situ DNA end labeling technique. Acta. Neurobiol. Exp. (Warsz) 58, 23–28.
Simonati A., Rosso T., and Rizzuto N. (1997) DNA fragmentation in normal development of the human central nervous system: a morphological study during corticogenesis. Neuropathol. Appl. Neurobiol. 23, 203–211.
Fekete D. M., Homburger S. A., Waring M. T., Riedl A. E., and Garcia L. F. (1997) Involvement of programmed cell death in morphogenesis of the vertebrate inner ear. Development 124, 2451–2461.
Vaahtokari A., Aberg T., and Thesleff I. (1996) Apoptosis in the developing tooth: association with an embryonic signaling center and suppression by EGF and FGF-4. Development 122, 121–129.
Hensey C. and Gautier J. (1998) Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev. Biol. 203, 36–48.
Asai K., Kudej R. K., Shen Y. T., Yang G. P., Takagi G., Kudej A. B., Geng Y. J., Sato N., Nazareno J. B., Vatner D. E., Natividad F., Bishop S. P., and Vatner S. F. (2000) Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler. Thromb. Vasc. Biol. 20, 1493–1499.
Borras D., Pumarola M., and Ferrer I. (2000) Neuronal nuclear DNA fragmentation in the aged canine brain: apoptosis or nuclear DNA fragility? Acta. Neuropathol. (Berl) 99, 402–408.
Savory J., Rao J. K., Huang Y., Letada P. R., and Herman M. M. (1999) Age-related hippocampal changes in Bcl-2:Bax ratio, oxidative stress, redox-active iron and apoptosis associated with aluminum-induced neurodegeneration: increased susceptibility with aging. Neurotoxicology 20, 805–817.
Aggarwal S., Gollapudi S., and Gupta S. (1999) Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J. Immunol. 162, 2154–2161.
Harocopos G. J., Alvares K. M., Kolker A. E., and Beebe D. C. (1998) Human age-related cataract and lens epithelial cell death. Invest. Ophthalmol. Vis. Sci. 39, 2696–2706.
Li W. P., Chan W. Y., Lai H. W., and Yew D. T. (1997) Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J. Mol. Neurosci. 8, 75–82.
Troncoso J. C., Sukhov R. R., Kawas C. H., and Koliatsos V. E. (1996) In situ labeling of dying cortical neurons in normal aging and in Alzheimer’s disease: correlations with senile plaques and disease progression. J. Neuropathol. Exp. Neurol. 55, 1134–1142.
Aggarwal S. and Gupta S. (1998) Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J. Immunol. 160, 1627–1637.
Usami S., Takumi Y., Fujita S., Shinkawa H., and Hosokawa M. (1997) Cell death in the inner ear associated with aging is apoptosis? Brain Res. 747, 147–150.
Kiatipattanasakul W., Nakamura S., Hossain M. M., Nakayama H., Uchino T., Shumiya S., Goto N., and Doi K. (1996) Apoptosis in the aged dog brain. Acta. Neuropathol. (Berl) 92, 242–248.
Foster J. R. (2000) Cell death and cell proliferation in the control of normal and neoplastic tissue growth. Toxicol. Pathol. 28, 441–446.
Kohji T., Hayashi M., Shioda K., Minagawa M., Morimatsu Y., Tamagawa K., and Oda M. (1998) Cerebellar neurodegeneration in human hereditary DNA repair disorders. Neurosci. Lett. 243, 133–136.
Sugawa M., Ikeda S., Kushima Y., Takashima Y., and Cynshi O. (1997) Oxidized low density lipoprotein caused CNS neuron cell death. Brain Res. 761, 165–172.
Heesters M. A., Koudstaal J., Go K. G., and Molenaar W. M. (1999) Analysis of proliferation and apoptosis in brain gliomas: prognostic and clinical value. J. Neurooncol. 44, 255–266.
Mundle S. D., Gao X. Z., Khan S., Gregory S. A., Preisler H. D., and Raza A. (1995) Two in situ labeling techniques reveal different patterns of DNA fragmentation during spontaneous apoptosis in vivo and induced apoptosis in vitro. Anti-cancer. Res. 15, 1895–1904.
Bodis S., Siziopikou K. P., Schnitt S. J., Harris J. R., and Fisher D. E. (1996) Extensive apoptosis in ductal carcinoma in situ of the breast. Cancer 77, 1831–1835.
Chia S. J., Tang W. Y., Elnatan J., Yap W. M., Goh H. S., and Smith D. R. (2000) Prostate tumours from an Asian population: examination of bax, bcl-2, p53 and ras and identification of bax as a prognostic marker. Br. J. Cancer 83, 761–768.
Yamasaki F., Tokunaga O., and Sugimori H. (1997) Apoptotic index in ovarian carcinoma: correlation with clinicopathologic factors and prognosis. Gynecol. Oncol. 66, 439–448.
Kiyozuka Y., Akamatsu T., Singh Y., Ichiyoshi H., Senzaki H., and Tsubura A. (1999) Optimal prefixation of cells to demonstrate apoptosis by the TUNEL method. Acta. Cytol. 43, 393–399.
Zhang X. and Takenaka I. (2000) Cell proliferation and apoptosis with BCL-2 expression in renal cell carcinoma. Urology 56, 510–515.
Hindermann W., Berndt A., Wunderlich H., Katenkamp D., and Kosmehl H. (1997) Quantitative evaluation of apoptosis and proliferation in renal cell carcinoma. Correlation to tumor subtype, cytological grade according to thoenes-classification and the occurrence of metastasis. Pathol. Res. Pract. 193, 1–7.
Thomas L. B., Gates D. J., Richfield E. K., O’Brien T. F., Schweitzer J. B., and Steindler D. A. (1995) DNA end labeling (TUNEL) in Huntington’s disease and other neuropathological conditions. Exp. Neurol. 133, 265–272.
Jellinger K. A. (2000) Cell death mechanisms in Parkinson’s disease. J. Neural. Transm. 107, 1–29.
He Y., Lee T., and Leong S. K. (2000) 6-Hydroxydopamine induced apoptosis of dopaminergic cells in the rat substantia nigra. Brain Res. 858, 163–166.
Kingsbury A. E., Mardsen C. D., and Foster O. J. (1998) DNA fragmentation in human substantia nigra: apoptosis or perimortem effect? Mov. Disord. 13, 877–884.
Kitt C. A. and Wilcox B. J. (1995) Preliminary evidence for neurodegenerative changes in the substantia nigra of Rett syndrome. Neuropediatrics 26, 114–118.
Anderson A. J., Stoltzner S., Lai F., Su J., and Nixon R. A. (2000) Morphological and biochemical assessment of DNA damage and apoptosis in Down syndrome and Alzheimer disease, and effect of postmortem tissue archival on TUNEL. Neurobiol. Aging. 21, 511–524.
Ekegren T., Grundstrom E., Lindholm D., and Aquilonius S. M. (1999) Upregulation of Bax protein and increased DNA degradation in ALS spinal cord motor neurons. Acta. Neurol. Scand. 100, 317–321.
Kerrigan L. A., Zack D. J., Quigley H. A., Smith S. D., and Pease M. E. (1997) TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch. Ophthalmol. 115, 1031–1035.
Smale G., Nichols N. R., Brady D. R., Finch C. E., and Horton W. E.,Jr. (1995) Evidence for apoptotic cell death in Alzheimer’s disease. Exp. Neurol. 133, 225–230.
Davies K. J. (1995) Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 61, 1–31.
Facchinetti F., Dawson V. L., and Dawson T. M. (1998) Free radicals as mediators of neuronal injury. Cell. Mol. Neurobiol. 18, 667–682.
Jenner P. and Olanow C. W. (1998) Understanding cell death in Parkinson’s disease. Ann. Neurol. 44, S72–84.
Nunomura A., Perry G., Pappolla M. A., Wade R., Hirai K., Chiba S., and Smith M. A. (1999) RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 19, 1959–1964.
Mecocci P., MacGarvey U., and Beal M. F. (1994) Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann. Neurol. 36, 747–751.
Mecocci P., Polidori M. C., Ingegni T., Cherubini A., Chionne F., Cecchetti R., and Senin U. (1998) Oxidative damage to DNA in lymphocytes from AD patients. Neurology 51, 1014–1017.
Zhang J., Perry G., Smith M. A., Robertson D., Olson S. J., Graham D. G., and Montine T. J. (1999) Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 154, 1423–1429.
Browne S. E., Bowling A. C., MacGarvey U., Baik M. J., Berger S. C., Muqit M. M., Bird E. D., and Beal M. F. (1997) Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann. Neurol. 41, 646–653.
Polidori M. C., Mecocci P., Browne S. E., Senin U., and Beal M. F. (1999) Oxidative damage to mitochondrial DNA in Huntington’s disease parietal cortex. Neurosci. Lett. 272, 53–56.
Ferrante R. J., Browne S. E., Shinobu L. A., Bowling A. C., Baik M. J., MacGarvey U., Kowall N. W., Brown R. H.,Jr., and Beal M. F. (1997) Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 69, 2064–2074.
Bogdanov M., Brown R. H., Matson W., Smart R., Hayden D., O’Donnell H., Flint Beal M., and Cudkowicz M. (2000) Increased oxidative damage to DNA in ALS patients [In Process Citation]. Free Radic. Biol. Med. 29, 652–658.
Goyal V. K. (1982) Lipofuscin pigment accumulation in human brain during aging. Exp. Gerontol. 17, 481–487.
Stojanovic A., Roher A. E., and Ball M. J. (1994) Quantitative analysis of lipofuscin and neurofibrillary tangles in the hippocampal neurons of Alzheimer disease brains. Dementia 5, 229–233.
Usachev Y. M., Khammanivong A., Campbell C., and Thayer S. A. (2000) Particle-mediated gene transfer to rat neurons in primary culture. Pflugers Arch. 439, 730–738.
Nunomura A., et al. (1999) RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 19, 1959–1964.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2002 Humana Press Inc.
About this protocol
Cite this protocol
Kalyuzhny, A.E. (2002). Simultaneous In Situ Detection of DNA Fragmentation and RNA/DNA Oxidative Damage Using TUNEL Assay and Immunohistochemical Labeling for 8-Hydroxy-2′-Deoxyguanosine (8-OHdG). In: Didenko, V.V. (eds) In Situ Detection of DNA Damage. Methods in Molecular Biology, vol 203. Humana Press. https://doi.org/10.1385/1-59259-179-5:219
Download citation
DOI: https://doi.org/10.1385/1-59259-179-5:219
Publisher Name: Humana Press
Print ISBN: 978-0-89603-952-0
Online ISBN: 978-1-59259-179-4
eBook Packages: Springer Protocols