Abstract
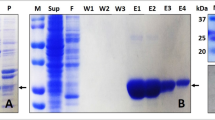
Water-soluble quinoprotein glucose dehydrogease (PQQGDH-B) is a dimeric enzyme whose application for glucose sensing is the focus of much attention. We attempted to increase the thermal stability of PQQGDH-B by introducing a disulfide bond at the dimer interface. The Ser residue at position 415 was selected for substitution with Cys, as structural information revealed that its side chains face each other at the dimer interface of PQQGDH-B. PQQGDH-B with Ser415Cys showed 30-fold greater thermal stability at 55°C than did the wild-type enzyme without any decrease in catalytic activity. After incubation at 70°C for 10 min, Ser415Cys retained 90% of the GDH activity of the wild-type enzyme. Disulfide bond formation between the mutant subunits was confirmed by analyses with sodium dodecylsulfate-polyacrylamide gel electrophoresis in the presence and absence of reductants. Our results indicate that the introduction of one Cys residue in each monomer of PQQGDH-B resulted in formation of a disulfide bond at the dimer interface and thus achieved a large increase in the thermal stability of the enzyme.
Similar content being viewed by others
References
D’Costa, E.J., Higgins, I.J., and Turner, A.P.F. (1986) Quinoprotein glucose dehydrogenase and its application in an amperometric glucose sensor. Biosensors, 2, 71–87.
Yokoyama, K., Sode, K., Tamiya, E., and Karube, I. (1989) Integrated biosensor for glucose and galactose. Anal. Chim. Acta. 218, 137–142.
Smolander, M., Livio, H. -L., and Rasanen, L. (1992) Mediated amperometric determination of xylose and glucose with an immobilized aldose dehydrogenase electrode. Biosensors Bioelectronics 7, 637–643.
Sode, K., Nakasono, S., Tanaka, M., and Matsunaga, T. (1993) Subzero temperature operating biosensor utilizing an organic solvent and quinoprotein glucose dehydrogenase. Biotechnol. Bioeng., 42, 251–254.
Ye, L., Hammerle, M., Olsthoorn, A.J.J., Schuhmann, W., Schmidt, H.-L., Duine, J.A., and Heller, A. (1993) High current density “wired” quinoprotein glucose dehydrogenase electrode. Anal. Chem. 65, 238–241.
Katz, E., Schlereth, D.D., and Schmidt, H.-L. (1994) Reconstitution of the quinoprotein glucose dehydrogenase from its apoenzyme on a gold electrode surface modified with a monolayer of pyrroloquinoline quinone. Electroanal. Chem. 368, 165–171.
Kost, G.J., Vu, H.-T., Lee, J.H., Bourgeois, P., Kiechle, F.L., Martin, C., et al. (1998) Multi-center study of oxygen-insensitive handheld glucose point-of-care testing in critical care/hospital/ambulatory patients in the United States and Canada. Crit. Care. Med. 26, 581–590.
Laurinavicius, V., Kurtinaitiene, B., Liauksminas, V., Jankauskaite, A., Simkus, R., Meskys, R., et al. (2000) Reagentless biosensor based on PQQ-dependent glucose dehydrogenase and partially hydrolyzed polyarbutin. Talanta 52, 485–493.
Razumiene, J., Meskys, R., Gureviciene, V., Laurinavicius, V., Reshetova, M.D. and Ryabov, A.D. (2000) 4-Ferrocenyllphenol as an electron transfer mediator on PQQ-dependent alcohol and glucose dehydrogenase-catalyzed reactions. Electrochem. Commun. 2, 307–311.
Schmidt, B. (1997) A new class of enzymes for application in diagnostics. Clinica. Chim. Acta. 26, 33.
Mullen, W.H., Churchhouse, J. and Vadgama, P. (1985) Enzyme electrode for glucose based on the quinoprotein glucose dehydrogenase. Analyst 110, 925.
Takahashi, Y., Igarashi, S., Nakazawa, Y., Tsugawa, W., and Sode, K. (2000) Construction and characterization of glucose enzyme sensor employing engineered water soluble PQQ glucose dehydrogenase with improved thermal stability. Electrochemistry 68, 907–911.
Sode, K. and Sano, H. (1994) Glu742 substitution to Lys enhanced the EDTA tolerance of Escherichia coli PQQ glucose dehydrogenase. Biotechnol. Lett., 16, 455–460.
Sode, K., Yoshida, H., Matsumura, K., Kikuchi, T., Watanabe, M., Yasutake, N., et al. (1995) Elucidation of the region responsible for EDTA tolerance in PQQ glucose dehydrogenases by constructing Escherichia coli and Acinetobacter calcoaceticus chimeric enzymes. Biochem. Biophys. Res. Commun. 211, 268–273.
Sode, K., Watanabe, K., Ito, S., Matsumura, K. and Kikuchi, T., (1995) Thermostable chimeric PQQ glucose dehydrogenase. FEBS Lett. 364, 325–327.
Sode, K. and Yoshida, H. (1997) Construction and characterization of a chimeric Escherichia coli PQQ glucose dehydrogenase (PQQGDH) with increased EDTA tolerance. Denki Kagaku. 65, 444–451.
Yoshida, H. and Sode, K. (1997) Thr424 to Asn substitution alters bivalent metal specificity of pyrroloquinoline quinone glucose dehydrogenase. J. Biochem. Mol. Biol. Biophys. 1, 89–93.
Sode, K. and Kojima, K. (1997) Improved substrate specificity and dynamic range for glucosse measurement of Escherichia coli PQQ glucose dehydrogenase by site directed mutagenesis. Biotechnol. Lett. 19, 1073–1077.
Yoshida, H., Kojima, K., Witarto, A.B., and Sode, K. (1999) Engineering a chimeric pyrroloquinoline quinone glucose dehydrogenase: improvement of EDTA tolerance, thermal stability and substrate specificity. Protein Eng. 12, 63–70.
Witarto, A.B., Ohuchi, S., Narita, M. and Sode, K. (1999) Secondary structure study of pyrroloquinoline quinone glucose dehydrogenase. J. Biochem. Mol. Biol. Biophys. 2, 209–213.
Witarto, A.B., Ohtera, T. and Sode, K. (1999) Site-directed mutagenesis study on the thermal stability of a chimeric PQQ glucose dehydrogenase and its structural interpretation. Appl. Biochem. Biotechnol. 77–79, 159–168.
Yoshida, H., Iguchi, T., Sode, K. (2000) Construction of mulit-chimeric pyrroloquinoline quinone glucose dehydrogenase with improved enzymatic properties and application in glucose monitoring Biotechnol. Lett. 22, 1505–1510.
Okuda, J., Yoshida, H., Kojima, K., Himi, M. and Sode, K. (2000) The role of conserved Hia775/781 in membrane-binding PQQ glucose dehydrogenase of Escherichia coli and Acinetobacter calcoaceticus. J. Biochem. Mol. Biol. Biophys. 4, 415–422.
Sode, K., Ohtera, T., Shirahane, M., Witarto, A.B., Igarashi, S., and Yoshida, H. (2000) Increasing the thermal stability of the water-soluble pyrroloquinoline quinone glucose dehydrogenase by single amino acid replacement. Enz. Microbial. Technol. 26, 491–496.
Sode, K., Shirahane, M., and Yoshida H. (1999) Construction and characterization of a linked-dimeric pyrrolquinoline quinone glucose dehydrogenase. Biotechnol. Lett. 21, 707–710.
Igarashi, S., Ohtera T., Yoshida, H., Witarto, A.B., and Sode, K. (1999) Construction and characterization of mutant water-soluble PQQ glucose dehydrogenases with altered Km value-site-directed mutagenesis studies on the putative active site. Biochem. Biophys. Res. Commun. 264, 820–824.
Geiger, O. and Görisch, H. (1989) Reversible thermal inactivation of the quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus. Biochem. J. 261, 415–421.
Sauer, R.T., Hehir, K., Stearman, R.S., Weiss, M.A., Jeitler-Nilsson, A., Suchanek E.G, et al. (1986) An engineered intersubunit disulfide enhances the stability and DNA binding on the N-terminal domain of lambda repressor. Biochemistry 7, 5992–5998.
Heikoop, J.C., van den Boogart, P., Mulders J.W.W., and Grootenhuis P.D.J. (1997) Structure-based design and protein engineering of intersubunit disulfide bonds in gonadotropins. Nat. Biotechnol. 15, 658–662.
Bunting, K.A., Cooper, J.B., Tickle, I.J., and Young, D.B. (2002) Engineering of an intersubunit disulfide bridge in the iron-superoxide dismutase of Mycobacterium tuberculosis. Arch. Biochem. Biophys. 397, 69–76.
Oubrie, A., Rozeboom, H.J., Kalk, K.H., Duine, J.A., and Dijkstra, B.W. (1999) The 1.7Å crystal structure of the apo-form of the soluble quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus reveals a novel internal conserved sequence repeat. J. Mol. Biol. 289, 319–333.
Oubrie, A., Rozeboom, H.J., Kalk, K.H., Olsthoorn, A.J.J., Duine, J.A., and Dijkstra B.W. (1999) Structure and mechanism of soluble quinoprotein glucose dehydrogenase. EMBO J. 18 5187–5194.
Oubrie, A., Rozeboom, H.J., and Dijkstra, B.W. (1999) Active-site structure of the soluble quino-protein glucose dehydrogenase complexed with methylhydrazine: A covalent cofactor-inhibitor complex. Proc. Natl. Acad. Sci. USA 96, 11787–11797.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Igarashi, S., Sode, K. Stabilization of quaternary structure of water-soluble quinoprotein glucose dehydrogenase. Mol Biotechnol 24, 97–103 (2003). https://doi.org/10.1385/MB:24:2:97
Issue Date:
DOI: https://doi.org/10.1385/MB:24:2:97