Abstract
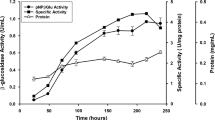
A β-glucosidase (BglA, EC 3.2.1.21) gene from the polycentric anaerobic fungus Orpinomyces PC-2 was cloned and sequenced. The enzyme containing 657 amino acid residues was homologous to certain animal, plant, and bacterial β-glucosidases but lacked significant similarity to those from aerobic fungi. Neither cellulose- nor protein-binding domains were found in BglA. When expressed in Saccharomyces cerevisiae, the enzyme was secreted in two forms with masses of about 110 kDa and also found in two forms associated with the yeast cells. K m and V max values of the secreted BglA were 0.762 mM and 8.20 µmol/(min·mg), respectively, with p-nitrophenyl-β-d-glucopyranoside (pNPG) as the substrate and 0.310 mM and 6.45 µmol/(min·mg), respectively, for the hydrolysis of cellobiose. Glucose competitively inhibited the hydrolysis of pNPG with a K i of 3.6 mM. β-Glucosidase significantly enhanced the conversion of cellulosic materials into glucose by Trichoderma reesei cellulase preparations, demonstrating its potential for use in biofuel and feedstock chemical production.
Similar content being viewed by others
References
Ohmiya, K., Sakka, K., Karita, S., and Kimura, T. (1997), Gen. Eng. Rev. 14, 365–414.
Filho, E. X. F. (1996), Can. J. Microbiol. 42, 1–5.
Chen, H., Li, X.-L., and Ljungdahl, L. G. (1994), Appl. Environ. Microbiol. 60, 64–70.
Borneman, W. S., Akin, D. E., and Ljungdahl, L. G. (1989), Appl. Environ. Microbiol. 55, 1066–1073.
Li, X.-L., Chen, H., and Ljungdahl, L. G. (1997), Appl. Environ. Microbiol. 63, 628–635.
Li, X.-L., Chen, H., and Ljungdahl, L. G. (1997), Appl. Environ. Microbiol. 63, 4721–4728.
Chen, H., Li, X.-L., Blum, D. L., and Ljungdahl, L. G. (1998), FEMS Microbiol. Lett. 159, 63–68.
Chen, H., Li, X.-L., Blum, D. L., Ximenes, E. A., and Ljungdahl, L. G. (2003), Appl. Biochem. Biotechnol. 105–108, 775–785.
Chen, H., Li, X.-L., and Ljungdahl, L. G. (1997), J. Bacteriol. 179, 6028–6034.
Blum, D. L., Li, X.-L., Chen, H., and Ljungdahl, L. G. (1999), Appl. Environ. Microbiol. 65, 3990–3995.
Chen, H., Li, X.-L., and Ljungdahl, L. G. (1995), Proc. Natl. Acad. Sci. USA 9, 2587–2591.
Herr, D., Baumer, F., and Dellweg, H. (1978), Appl. Microbiol. Biotechnol. 5, 29–36.
Chen, H., Ximenes, E. A., Li, X.-L., and Ljungdahl, L. G. (1999), in Cellulose Degradation, Ohmiya, K., Hayashi, K., Sakka, K., Kobayashi, Y., Karita, S., and Kimura, T., eds., Uni Publishers, Tokyo, Japan, pp. 173–181.
Laemmli, U. K. (1970), Nature (Lond.) 227, 680–685.
Fairbanks, G., Steak, T. S., and Wallach, D. F. H. (1971), Biochemistry 10, 2606–2616.
Rutenburg, A. M., Goldbarg, J. A., Rutenburg, S. H., and Lang, R. T. (1960), J. Histochem. Cytochem. 8, 268–272.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956), Anal. Chem. 28, 350–356.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951), J. Biol. Chem. 193, 265–273.
Miller, G. L. (1959), Anal. Chem. 31, 426–428.
Li, X.-L., Chen, H., He, Y., Blum, D. L., and Ljungdahl, L. G. (1997), in Abstracts of 97 th General Meeting of the American Society for Microbiology, American Society for Microbiology, Washington, DC, p. 424.
Steenbakkers, P. J. M., Harhangi, H. R., Bosscher, M. W., van der Hooft, M. M. C., Keltjens, J. T., van der Drift, C., Vogels, G. D., and Op den Camp, H. J. M. (2003), Biochem. J. 370, 963–970.
Steenbakkers, P. J. M., Li, X.-L., Ximenes, E. A., Arts, J. G., Chen, H., Ljungdahl, G. L., and Op den Camp, H. J. M. (2001), J. Bacteriol. 183, 5325–5333.
Bǵuin, P. and Lemaire, M. (1996), Crit. Rev. Biochem. Mol. Biol. 31, 201–236.
Fannuti, G., Ponyi, T., Black, G. W., Hazlewood, G. P., and Gilbert, H. J. (1995), J. Biol. Chem. 270, 29,314–29,322.
von Heijne, G. (1986), Nucleic Acids Res. 14, 4683–4690.
Harhangi, H. R., Steenbakkers, P. J. M., Akmanova, A., Jetten, M. S. M., van der Drift, C., and Op den Camp, H. J. M. (2002), Biochem. Biophys. Acta 1574, 293–303.
Hays, W. S., Jenison, S. A., Yamada, T., Pastuszyn, A., and Glew, R. H. (1996), Biochem. J. 319, 829–837.
Inoue, M., Shibuya, M., Yamamoto, K., and Ebizuka, Y. (1996), FEBS Lett. 389, 273–277.
Grbnitz, F., Seiss, M., Rüknagel, K. P., and Staudenbauer, W. L. (1991), Eur. J. Biochem. 200, 301–309.
Paavilainen, S., Hellman, J., and Korpela, T. (1993), Appl. Environ. Microbiol. 59, 927–932.
Breves, R., Bronnenmeier, K., Wild, N., Lottspeich, F., Staudenbauer, W. L., and Hofemeister, J. (1997), Appl. Environ. Microbiol. 63, 3902–3910.
Liebl, W., Gabelsberger, J., and Schleifer, K.-H. (1994), Mol. Gen. Genet. 242, 111–115.
Henrissat, B. and Bairoch, A. (1993), Biochem. J. 293, 781–788.
Sanz-Aparicio, J., Hermoso, J. A., Martinez-Ripoll, M., Lequerica, J. L., and Polaina, J. (1998), J. Mol. Biol. 275, 491–502.
Penttilä, M. E., André, L., Saloheimo, M., Lehtovaara, P., and Knowles, J. K. C. (1987), Yeast 3, 175–185.
Penttilä, M. E., André, L., Lehtovaara, P., Bailey, M., Teeri, T. T., and Knowles, J. K. C. (1988), Gene 63, 103–112.
Cummings, C. and Fowler, T. (1996), Curr. Genet. 29, 227–233.
Li, X.-L. and Ljungdahl, L. G. (1996), Appl. Environ. Microbiol. 62, 209–213.
Rothstein, S. J., Lanhners, K. N., Lazarus, C. M., Baulcombe, D. C., and Gatenby, A. A. (1987), Gene 55, 353–356.
van Rensburg, P., Van Zyl, W. H., and Pretorius, I. S. (1998), Yeast 14, 67–76.
Ngsee, J. K., Hansen, W., Walter, P., and Smith, M. (1989), Mol. Cell. Biol. 9, 3400–3410.
Orlean, P., Kuranda, M. J., and Albright, C. F. (1991), Methods Enzymol. 194, 682–696.
Herbaud, M. and Fevre, M. (1990), Appl. Environ. Microbiol. 56, 3164–3169.
Teunissen, M. J., Lahaye, D. H. T. P., Huis In’t Veld, J. H. J., and Vogels, G. D. (1992), Arch. Microbiol. 158, 276–281.
Li, X.-L. and Calza, R. E. (1991), Enzyme Microb. Technol. 13, 622–628.
Li, X.-L. and Calza, R. E. (1991), Biochem. Biophys. Acta 1080, 148–154.
Ward, M. (1989), in EMBO-ALKO Workshop on Molecular Biology of Filamentous Fungi, Nevalainen, H. and Pentillä, M., eds., Foundation for Biotechnical and Industrial Fermentation Research, Espoo, Finland, pp. 119–128.
Archer, D. B. and Peberdy, J. F. (1997), Crit. Rev. Biotechnol. 17, 273–306.
Ohmiya, K., Shirai, M., Kurachi, Y., and Shimizi, S. (1985), J. Bacteriol. 161, 432–434.
Author information
Authors and Affiliations
Corresponding author
Additional information
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may be suitable.
Rights and permissions
About this article
Cite this article
Li, XL., Ljungdahl, L.G., Ximenes, E.A. et al. Properties of a recombinant β-glucosidase from polycentric anaerobic fungus Orpinomyces PC-2 and its application for cellulose hydrolysis. Appl Biochem Biotechnol 113, 233–250 (2004). https://doi.org/10.1385/ABAB:113:1-3:233
Issue Date:
DOI: https://doi.org/10.1385/ABAB:113:1-3:233