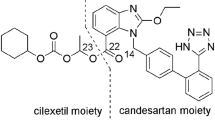
Abstract
Five impurities were observed when candesartan cilexetil tablets were subjected to stability and forced degradation studies. These impurities were successfully isolated and characterized as desethyl candesartan cilexetil, 1N-ethyl candesartan cilexetil, 2N-ethyl candesartan cilexetil, 1N-ethyl oxo candesartan cilexetil, and 2N-ethyl oxo candesartan cilexetil. A gradient reverse phase liquid chromatography (LC) and an isocratic preparative LC method were used to detect and isolate all five degradation products impurities simultaneously. Mass spectrometry, 1H/13C, DEPT and 2D NMR experiments were extensively utilized to characterize these impurities. Even though desethyl candesartan cilexetil, 1N-ethyl candesartan cilexetil were 2N-ethyl candesartan cilexetil were documented in the literature as known impurities, the regioisomers 1N-ethyl oxo candesartan cilexetil and 2N-ethyl oxo candesartan cilexetil were never noticed. Single-crystal diffraction data has been used to confirm their structure unambiguously and synthetic preparations of all known and unknown impurities were also presented.





Similar content being viewed by others
References
Michihiro Y, Hirofumi Y (2000) Clin Pharmacol 93:175–182
Kenichi M, Shinji I, Keiko M, Shigeru E, Masayuki T, Mamoru O, Hideki S, Masahiko K (1998) Cardiovasc Drugs Ther 12:469–474. doi:10.1023/A:1007754100351
Stenhoff H, Lagerström P-O, Andersen C (1999) J Chromatogr B Analyt Technol Biomed Life Sci 731:411–417. doi:10.1016/S0378-4347(99)00247-9
Ferreirós N, Dresen S, Alonso RM, Weinmann W (2007) J Chromatogr B Analyt Technol Biomed Life Sci 855:134–138. doi:10.1016/j.jchromb.2007.04.009
Subba Rao DV, Radhakrishnanand P, Suryanarayana MV, Himabindu V (2007) Chromatographia 66:499–507. doi:10.1365/s10337-007-0364-x
International Conference on Harmonization guideline (2006) Impurities in New Drug Substances Q3A (R2)
International Conference on Harmonization guideline (2006) Impurities in New Drug products Q3B (R2)
International Conference on Harmonization (1995) ICH guidelines on validation of analytical procedures: text and methodology Q2 (R1): FDA. Fed Regist 60:11260
Kurgan Z, Pesachovich M (2006) World intellectual property organization, WO 2006/122254 A2
Holzer W, Jäger C (1992) Monatsh Chem 123:1027–1036. doi:10.1007/BF00810934
Acknowledgments
The authors wish to thank the management of the Torrent Research Center for allowing us to carry out the present work. The authors are also grateful to Dr. C. Dutt, Director, Dr. Sunil S. Nadkarani, Vice President and Mr. P. C. Gandhi, Vice President of Torrent Research Center for their constant encouragement. The authors also wish to thank friends and the other colleagues of the Torrent Research Center for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohan, A., Shanmugavel, S., Goyal, A. et al. Identification, Isolation, and Characterization of Five Potential Degradation Impurities in Candesartan Cilexetil Tablets. Chroma 69, 1211–1220 (2009). https://doi.org/10.1365/s10337-009-1066-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-009-1066-3