Abstract
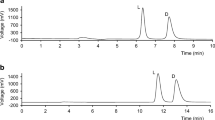
A normal phase chiral LC method for chiral purity evaluation of β-amino-β-(4-methoxyphenyl) propionic acid was developed on donor–acceptor (pirkle) column. The chiral stationary phase used was a 250 × 4.6 mm (R, R) Whelk-01 with 5 μm particle size, which was accompanied with a 1 cm long guard column. The “hybrid” pi-electron donor–acceptor based stationary phase (R, R) Whelk-01 was found to be enantiomeric selective for (R) and (S) enantiomers of β-amino-β-(4-methoxyphenyl) propionic acid with a resolution >2.5. The concentration of 2-propanol and TFA in the mobile phase plays an important role on the chrmatographic efficiency and resolution between the enantiomers. The limit of detection and limit of quantification of (S) enantiomer was 0.3 and 1.0 μg mL-1 for 20 μL injection volume. The percentage RSD of the peak area of six replicate injections of (S) enantiomer at LOQ concentration was 4.5. The percentage recovery of (S) enantiomer from (R) enantiomer samples ranged from 92 to 100. The test solution was observed to be stable up to 24 h after the preparation. The developed method was also checked by different analysts and on different lots of columns, reagents and it was proved to be rugged. The developed normal phase chiral LC method can be used for the determination of the enantiomeric purity of R-β-amino-β-(4-methoxyphenyl) propionic acid.




Similar content being viewed by others
References
Soloshonok VA, Fokina NA, Rybakova AV, Shishkina IP, Galushko SV, Sorochinsky AE, Kukhar VP, Savchenko MV, Svedas VK (1995) Tetrahedron Asymmetry 6:1601–1610
Tan CYK, Weaver DF (2002) Tetrahedron Lett 58:7449–7461
Davankov VA (1997) Pure Appl Chem 69:1469–1474
Berkecz R, Sztojkov-Ivanov A, Ilisz I, Forro E, Fulop F, Ho Hyun M, Peter A (2006) J Chromatogr A 1125:138–143
Snyder L, Kirkland JJ, Glajch J (1997) Practical HPLC method development, 2nd edn. Willey-Interscience Publishers, New York
United States Pharmacopiea (2004) Asian edition <621>, <1225>
Miller JM, Crowther JB (2000) Analytical chemistry in a GMP environment, pp 436
International conference on harmonization October (1994) Text on validation of analytical procedures Q2A
International conference on harmonization November (1996) Validation of analytical procedures methodology Q2B
Bakshi M, Singh S (2002) J Pharm Biomed Anal 28:1011–1040
Rao BM, Srinivasu MK,Balamurali K, Acharyulu PVR, Kumar RP, Chandrashekar KB (2005) Indian Drugs 42(5)
Acknowledgments
The authors wish to thank the management of Dr. Reddy’s group for supporting this work (part of my Ph.D. thesis work). The authors wish to acknowledge the Process Research group for providing the samples to carry out the above work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhavan, P., Rao, B.M., Pravin et al. A Validated Chiral HPLC Method for the Determination of Enantiomeric Purity of R-β-amino-β-(4-methoxyphenyl) Propionic Acid. Chroma 65, 81–84 (2007). https://doi.org/10.1365/s10337-006-0110-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0110-9