-
PDF
- Split View
-
Views
-
Cite
Cite
Chih‐Hsin Tang, Rong‐Sen Yang, Houng‐Chi Liou, Wen‐Mei Fu, Enhancement of Fibronectin Synthesis and Fibrillogenesis by BMP‐4 in Cultured Rat Osteoblast, Journal of Bone and Mineral Research, Volume 18, Issue 3, 1 March 2003, Pages 502–511, https://doi.org/10.1359/jbmr.2003.18.3.502
Close - Share Icon Share
Abstract
The skeletal extracellular matrix produced by osteoblasts contains the glycoprotein fibronectin (Fn), which regulates the adhesion, differentiation, and function of osteoblasts. Fn fibrillogenesis is involved in the process of bone mineralization. Bone morphogenetic proteins (BMPs) can be isolated from organic bone matrix and are able to initiate de novo cartilage and bone formation. In this study, the effect of BMP‐4 on Fn fibrillogenesis in cultured rat osteoblasts was examined. BMP‐4 enhanced Fn synthesis and extracellular Fn assembly in primary cultured osteoblasts. In addition, the extracellular assembly of Fn from exogenously applied soluble human Fn was also increased by BMP‐4. It has been reported that α5β1 integrin is related to Fn fibrillogenesis. The synthesis of both α5 and β1 integrins was upregulated by BMP‐4. Immunocytochemistry showed that the clustering of α5 and β1 integrins was also increased by BMP‐4. BMP‐4 increased fibril formation of Fn and the adhesion of osteoblasts onto Fn matrix, which was inhibited by disintegrin triflavin and Gly‐Arg‐Gly‐Asp‐Ser (GRGDS) peptide. Phosphorylation of extracellular signal‐regulated kinase (ERK) and focal adhesion kinase (FAK) was increased by BMP‐4. Enhancement of extracellular Fn fibrillogenesis and the mRNA expression of β1 integrin by BMP‐4 were inhibited by ERK kinase (MEK) inhibitor PD98059. These results suggest that the enhancement of extracellular Fn fibrillogenesis by BMP‐4 in cultured osteoblasts is related to the increase of the synthesis of Fn and clustering of α5 and β1 integrins. ERK is involved in the signaling pathway of BMP‐4 in regulating Fn fibrillogenesis in osteoblasts.
INTRODUCTION
THE EXTRACELLULAR MATRIX (ECM) provides positional and environmental information that is essential for tissue function. The ECMs produced by osteoblasts are complex and consist of several different classes of molecules that may regulate the modeling and remodeling of bone. The ECMs also serve as a reservoir for growth factors, including members of the transforming growth factor‐β (TGF‐β) and fibroblast growth factor (FGF) superfamily.(1,2) Acting either alone or together, these components of the ECM produced by osteoblasts may subsequently regulate the cell adhesion, migration, proliferation, differentiation, survival, as well as the rate of bone formation.
Fibronectin (Fn) is an extracellular matrix component that is also present as a soluble protein in plasma and other body fluids.(3) The matrix form of Fn is believed to support cell adhesion, migration during embryogenesis, tumor growth, wound healing, angiogenesis, and inflammation.(4) Assembly of soluble Fn into matrix is a multistep process under cellular control.(5) Among the membrane components implicated in Fn matrix assembly, integrins have been demonstrated to have a central role.(6,7) Integrins, composed of α and β subunits, are a family of transmembrane receptors mediating adhesion to both ECM and cell surface molecules.(8,9) The specific adhesion depends on the interaction between the cell‐binding domain of Fn and cell surface integrin receptors. However, the mechanisms regarding how integrins modulate Fn assembly are not well understood. Transfection of α5 integrin and expression of α5β1‐integrin by Chinese hamster ovary (CHO) cells results in a large increase in Fn assembly, whereas α5‐deficient CHO B2 cells failed to assemble plasma Fn into ECM.(6,10)
Osteoblast differentiation is an essential part of bone formation, because active osteoblasts should be recruited at the site of osteoclastic bone resorption to compensate the continuous loss of bone matrix and maintain structural integrity of skeletal system. The biology of this process is also of considerable interest when applying therapies to promote bone repair after injury or during disease processes. Accumulating evidence indicates that several factors, such as hormones, cytokines, and growth factors, are involved in the osteoblastic differentiation.(11,12) Bone morphogenetic proteins (BMPs), with more than 20 members, belong to the TGF‐β superfamily and were originally identified by their unique ability to induce ectopic cartilage and bone formation in vivo.(12,13) It has been shown that BMP‐2 and BMP‐4 are synthesized by osteoblasts.(14) BMPs play important roles in bone formation and bone cell differentiation by stimulating alkaline phosphatase activity and synthesis of proteoglycan, collagen, and osteocalcin.(12,15) The BMP‐4 is a BMP‐2 subfamily and induces apoptosis in different cells.(16,17) Mechanical stress induces osteoblast differentiation, which then leads to osteogenesis.(18,19) This osteoblast differentiation seemed to be accompanied by an increase in BMP‐4 gene expression. Although BMP‐4 expression is upregulated under tensile stress or during distraction osteogenesis,(18,19) the exact role of BMP‐4 in these systems is still unclear.
ECMs and growth factors are likely to act cooperatively to stimulate osteoblast differentiation, as has been shown for other cell types.(20) There is strong evidence to suggest a role for Fn in the early stages of osteogenesis. The distribution of Fn in areas of skeletogenesis suggests that it may be involved in early stages of bone formation.(21,22) However, the effect of BMPs on Fn fibrillogenesis in osteoblasts have been mostly unknown. We here found that BMP‐4 enhanced Fn fibrillogenesis of osteoblasts by increasing the synthesis and assembly of Fn. Furthermore, the increase of synthesis and clustering of α5 and β1 integrins is involved in the mechanism of action of BMP‐4.
MATERIALS AND METHODS
Primary osteoblast cultures
The primary osteoblastic cells were obtained from the calvaria of the 18‐day‐old fetal rats. In brief, the pregnant rats were put in anesthesia using ip injection of pentobarbital (50 mg/kg). The calvaria of fetal rats were then dissected from fetal rats with aseptic technique. The soft tissues were removed under dissecting microscope. The calvaria were divided into small pieces and were treated with 0.1% collagenase (Sigma Chemical, St. Louis, MO, USA) solution for 10 minutes at 37°. The next two 20‐minute sequential collagenase digestions were then pooled and filtered through 70‐μm nylon filters (Falcon; BD BioSciences, San Jose, CA, USA). The cells were then grown on the plastic cell culture dishes in 95% air‐5% CO2 with Dulbecco's modified Ca2+‐free Eagle's medium (DMEM) (Gibco, Grand Island, NY, USA), which was supplemented with 20 mM HEPES and 10% heat‐inactivated FCS, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml) (pH adjusted to 7.6). The cell medium was changed twice a week. The characteristics of osteoblasts were confirmed by the morphology and the expression of alkaline phosphatase (ALP).
Immunocytochemistry
Osteoblasts were grown on glass coverslips. Cultures were rinsed once with PBS and fixed for 15 minutes at room temperature in phosphate buffer containing 4% paraformaldehyde. Cells were rinsed three times with PBS. After blocking with 4% bovine serum albumin (BSA) for 15 minutes, cells were incubated with rabbit anti‐rat Fn (1:100; Gibco‐BRL, Gaithersburg, MD, USA) for 1 h at room temperature. Cells were then washed again and labeled with FITC‐conjugated goat anti‐rabbit IgG (1:150; Leinco Tec. Inc., St. Louis, MO, USA) for 1 h. Finally, cells were washed, mounted, and examined with a Zeiss confocal microscope (LSM 410; Zeiss, Jena, Germany) as soon as possible. The mean fluorescence under 10‐15 cells was measured using a Zeiss confocal microscope (LSM 410). The adjustment value for image contrast and background offset of confocal microscope was fixed so that the variation of the relative fluorescence of control experiments is rather small. When the α5 or β1 integrin was examined, the cells were fixed with acetone for 30 s. After fixation, cells were washed with PBS and incubated with 4% BSA for 1 h. Cells were then incubated with rabbit anti‐rat α5 (1:500) or β1 integrin (1:100; Chemicon, Temecula, CA, USA) for 3 h and FITC‐conjugated goat anti‐rabbit IgG for 1 h at room temperature.
To observe Fn assembly apart from Fn synthesis by rat osteoblasts, human soluble Fn (30 μg/ml) was added to the cultures for overnight. Rat osteoblasts also used the exogenous human soluble Fn to form fibrillar Fn underneath the cells. After washout of residual soluble Fn, fixation, and blocking with 4% BSA for 15 minutes, cells were incubated for 1 h at room temperature with mouse anti‐human Fn (1:50; Transduction Laboratory, Lexington, KY, USA), which does not recognize endogenously released rat Fn. Cells were then washed again and labeled with FITC‐conjugated goat anti‐mouse IgG (1:150; Jackson ImmunoResearch, Baltimore, MD, USA) for 1 h. Finally, cells were washed, mounted, and examined with a Zeiss fluorescence microscope.
Flow cytometric analysis
Osteoblasts were plated in 6‐well (35‐mm) dishes. The cells were then washed with PBS and detached with trypsin at 37°. Cells were fixed for 10 minutes in PBS containing 1% paraformaldehyde. After rinsing in PBS, the cells were incubated with rabbit anti‐rat α2, α3, α4, α5, or β1 integrin antibody (1:100; Chemicon) for 1 h at 4°C. Cells were then washed again and incubated with FITC‐conjugated secondary IgG for 45 minutes and analyzed by flow cytometry using FACS Calibur (CellQuest software; Perkin Elmer, San Jose, CA, USA).
Western blotting analysis
Osteoblasts were plated on 6‐well (35‐mm) dishes. Cells were incubated with BMP‐4 for different time intervals as indicated in results and then washed with PBS and lysed for 30 minutes at 4° with RIPA buffer. Equal protein was applied per lane, and electrophoresis was performed under denaturing conditions on a 7.5% SDS gel and transferred to an Immobilon polyvinyldifluoride (PVDF) membranes at 4° overnight. The blots were blocked with 4% BSA for 1 h at room temperature and then probed with rabbit anti‐rat antibodies against Fn (1:1500), α5 (1:100), or β1 integrin (1:500) or mouse anti‐rat phosphorylated extracellular signal‐regulated kinase (pERK; 1:1000; Santa Cruz Technology, Santa Cruz, CA, USA) for 1 h at room temperature. After three washes, the blots were subsequently incubated with a donkey anti‐rabbit or sheep anti‐mouse peroxidase‐conjugated secondary antibody (1:2000; Amersham Pharmacia, Piscataway, NJ, USA) for 1 h at room temperature. The blots were visualized by enhanced chemiluminescence using Kodak X‐OMAT LS film (Kodak, Rochester, NY, USA). For normalization purposes, the same blot was also probed with mouse anti‐rat α‐tubulin antibody (1:1000; Oncogene, Boston, MA, USA). For immunoprecipitation, cell lysates were incubated with rabbit anti‐rat focal adhesion kinase (FAK) antibody (Santa Cruz Technology) and protein A/G (Santa Cruz Technology) for 12 h at 4°. The pelleted proteins were washed with RIPA buffer, denatured, and then fractionated by 8% SDS‐PAGE and transferred to PVDF membrane. The membranes were blocked in 4% BSA and incubated with mouse anti‐rat phospho‐tyrosine antibody (1:1000; Santa Cruz Technology) for 1 h at room temperature. The subsequent procedures followed those as mentioned above.
Cell adhesion assay
Plates with 96 wells (Costar) were coated overnight with 100 μl of Fn (30 μg/ml) or 1% (wt/vol) BSA in PBS at 4°. Osteoblasts collected from a culture near confluence were resuspended in DMEM and labeled with 2′,7′‐bis‐(2‐carboxyethyl)‐5‐(6)‐carboyfluorescein acetoxymethyl ester (BCECF/AM; 10 μg/ml) for 30 minutes at 37°. The labeled cells were washed and resuspended in DMEM to a density of 5 × 105/ml. A 90‐μl sample of the resuspended cells was incubated with 10 μl PBS or various concentrations of BMP‐4 for 30 minutes at 37°. Control or BMP‐4‐pretreated cells were applied to the Fn‐precoated 96‐well plates and incubated for 90 minutes at 37° to allow adhesion. After being washed twice with PBS, the nonadherent cells were removed by aspiration, and the 96‐well plates were read with a CytoFluor 2300 fluorescence plate reader (Millipore, Bedford, MA, USA).
mRNA analysis by reverse transcriptase‐polymerase chain reaction
Total RNA was extracted from osteoblasts using a TRIzol kit (MDBio Inc., Taipei, Taiwan). RNA (500 ng) from osteoblasts was used for reverse transcriptase polymerase chain reaction (RT‐PCR) using One‐Step RT‐PCR kit (Clontech, CA, USA). Amplification was accomplished with 17 cycles, which was within the linear change of PCR product (Biometra, Gottingen, Germany). PCR products were then separated electrophoretically in a 2% agarose DNA gel and stained with ethidium bromide. mRNA levels of β1 integrin were normalized to levels of β‐actin. The PCR primers used were as follows: β1 integrin, forward primer, GGACAGGAGAAAATGGACGA; reverse primer, TCTGACCATTTGTCGCTACG; β‐actin, forward primer, TGTCACCAACTGGGACGATA; reverse primer, TCTCCGGAGTCCATCACAAT.
Materials
The following materials were used in experiments: BCECF/AM (Molecular Probes, Eugene, OR, USA), Gly‐Arg‐Gly‐Asp‐Ser (GRGDS) Peptide Institute, Osaka, Japan). PD 98059 (RBI, Natick, MA, USA). TGF‐β1, recombinant human BMP‐2, BMP‐4, and BMP‐7 (R&D Systems Inc., Minneapolis, MN, USA). Triflavin was purified from Trimeresurus flavoviridis snake venom(23) and was kindly provided by Dr. Huang.
The values given are mean ± SE. The significance of difference between the experimental groups and control was assessed by Student's t‐test. The difference is significant if p < 0.05.
All protocols complied with institutional guidelines and were approved by Animal Care Committees of Medical College, National Taiwan University.
RESULTS
BMP‐4 enhanced Fn fibril formation of cultured osteoblasts
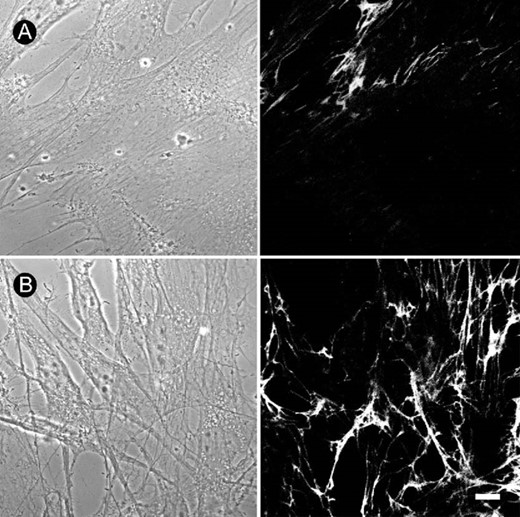
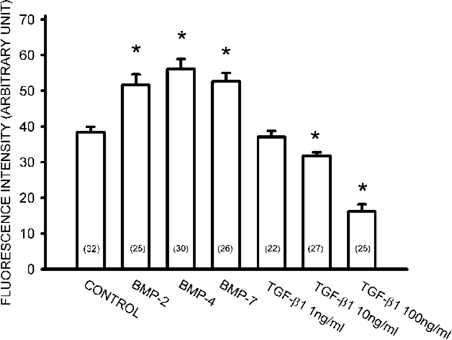
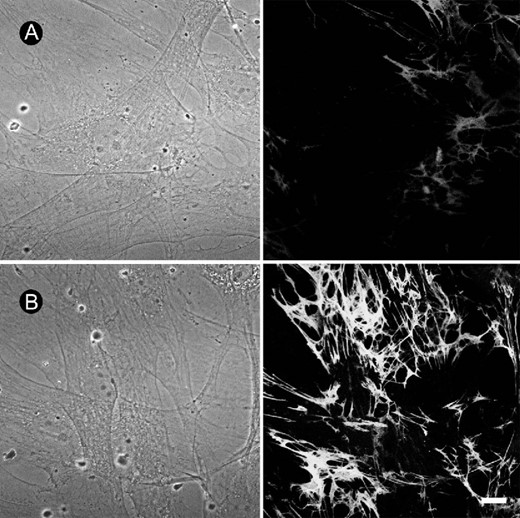
The fibrillogenesis from the endogenously released Fn by the primary cultured rat osteoblasts was studied using immunocytochemistry. Day 3 to day 5 osteoblasts were changed to serum‐free medium and incubated with BMP‐4 (20 ng/ml) for 12 h. The mean immunofluorescence intensity underneath a cell group of 10‐15 cells was measured using confocal microscope. As shown in Fig. 1A, osteoblasts are able to form Fn network underneath the cell using endogenously released Fn. Fn fibril formation increased in response to the treatment of BMP‐4 for 12 h (Fig. 1B). The mean fluorescence intensity underneath 10‐15 cells was 36.4 ± 2.5 and 63.3 ± 4.1 (n = 23‐28) for control and BMP‐4‐treated cells, respectively. We also examined the effect of other species of BMP on the Fn fibrillogenesis. As shown in Fig. 2, BMP‐2 and BMP‐7 (20 ng/ml each) also increased Fn fibrillogenesis, whereas TGF‐β1 dose‐dependently decreased Fn assembly. These results suggest that the action of BMPs is different from TGF‐β1 on the assembly of Fn. We investigated the action mechanism of BMP‐4 on Fn fibrillogenesis in the following experiments.
Increase of Fn fibrillogenesis by BMP‐4 in cultured rat osteoblasts. Fn network, which was shown by immunofluorescence, formed underneath the cultured osteoblasts. Compared with (A) control, (B) treatment with BMP‐4 (20 ng/ml) for 12 h increased Fn fibrillogenesis in cultured osteoblasts. Phase‐contrast images are shown in the left panels. Bar = 10 μm.
Differential regulation of Fn fibrillogenesis by BMPs and TGF‐β1. Fn network was shown by immunofluorescence and the mean fluorescence under 10‐15 cells was measured using a Zeiss confocal microscope. Note that treatment with BMP‐2, BMP‐4, and BMP‐7 (20 ng/ml each) for 12 h increased Fn fibrillogenesis, whereas TGF‐β1 dose‐dependently decreased Fn assembly. Data are presented as mean ± SE (n). *p < 0.05 compared with control.
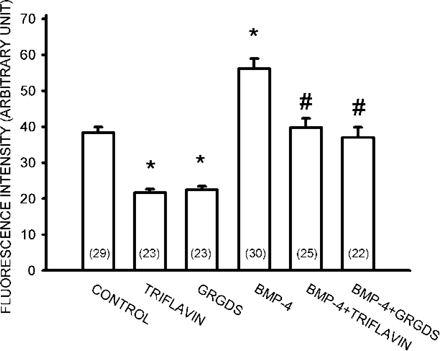
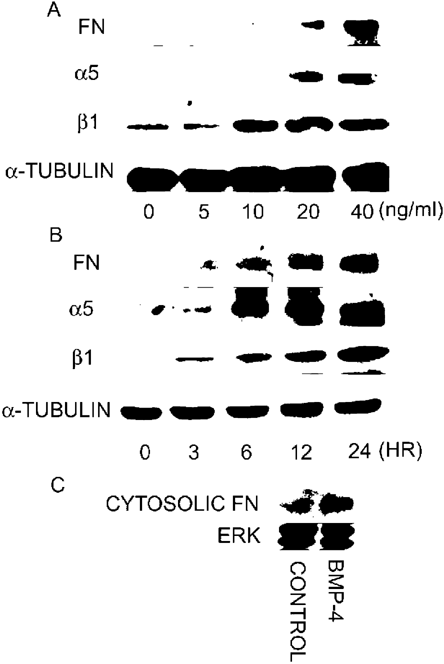
The assembly of extracellular Fn matrix underneath the cells may be related to integrins.(6,24) Integrins are a family of heterodimeric transmembrane receptors that contains α and β subunits. The different combination of α and β chains forms different receptors for the various kinds of ECM molecules. α5β1 integrin is a specific receptor for Fn. Application of GRGDS (50 μg/ml) or triflavin (2.8 μM), an Arg‐Gly‐Asp (RGD)‐dependent disintegrin,(23) inhibited Fn assembly and the enhancing effect of BMP‐4, indicating that RGD motif is involved in Fn fibrillogenesis (Fig. 3). Western blotting was used to examine the effect of BMP‐4 on the protein levels of Fn. Day 3 to day 5 osteoblasts were changed to serum‐free cultured medium and treated with BMP‐4 for 12 h. The cultures were then washed with cold PBS and protein samples were collected by the addition of lysis buffer. The result from Western blotting may contain both soluble cytosolic Fn and extracellular immobilized Fn. As shown in Fig. 4A, BMP‐4 dose‐dependently increased protein levels of Fn, α5, and β1 integrins after incubation with cells for 12 h. In addition, BMP‐4 also time‐dependently enhanced protein levels of Fn, α5, and β1 integrins when examined at a concentration of 20 ng/ml (Fig. 4B). We further quantified cytosolic soluble Fn levels by the digestion of the cells with trypsin‐EDTA (0.25% trypsin, 1 mM EDTA) for 2 minutes to get cytosolic fraction of the cell suspension after treatment with BMP‐4 for 12 h. As shown in Fig. 4C, BMP‐4 treatment (20 ng/ml) also increased cytosolic Fn. These results indicate that BMP‐4 may increase Fn fibrillogenesis by upregulating both Fn synthesis and extracellular Fn assembly.
Antagonism of BMP‐4 effect by disintegrin. Fn network was shown by immunofluorescence and the mean fluorescence under 10‐15 cells was measured using Zeiss confocal microscope. The cultured cells were incubated with various drugs for 12 h. Note that concomitant treatment with GRGDS or triflavin antagonized the Fn fibrillogenesis enhancing effect of BMP‐4. Data are presented as mean ± SE (n). *p < 0.05 compared with control. #p < 0.05 compared with BMP‐4‐treated group.
Upregulation of protein levels of Fn, α5, and β1 integrins by BMP‐4. (A) Osteoblast cultures were treated with different concentrations of BMP‐4 for 12 h. The cultures were washed with cold PBS, and protein samples for Western blotting analysis were collected by the direct addition of lysis buffer to cultures without trypsin digestion. Compared with control, BMP‐4 dose‐dependently increased the protein levels of Fn, α5, and β1 integrins. (B) Compared with control, BMP‐4 time‐dependently increased the protein levels of Fn, α5, and β1 integrins at a concentration of 20 ng/ml. (C) Cytosolic soluble Fn levels were measured by obtaining cytosolic fraction of the cultured cells after treatment with BMP‐4 for 12 h. Note that BMP‐4 also increased cytosolic Fn.
Effect of BMP‐4 on Fn assembly derived from exogenously applied soluble Fn
Increase of Fn fibril formation by BMP‐4 may result from the effect on Fn synthesis and/or extracellular Fn assembly. To simply look at the effect of BMP‐4 on Fn assembly apart from Fn synthesis, soluble human Fn (30 μg/ml) was applied into cultured medium for 12 h in the presence or absence of BMP‐4 (20 ng/ml). The cultures were then washed with plain culture medium to remove the remaining soluble form of human Fn and immunocytochemistry of immobilized Fn was performed using mouse anti‐human Fn monoclonal antibody, which does not recognize endogenously‐released rat Fn. Compared with control, BMP‐4 markedly enhanced Fn fibril formation from exogenously applied soluble Fn (Fig. 5). The mean fluorescence intensity underneath 10 to 15 cells was 30.4 ± 2.9 and 59.1 ± 3.7 (n = 25‐33) for control and BMP‐4‐treated groups, respectively.
BMP‐4 increased extracellular Fn assembly from exogenously applied soluble Fn. Culture medium of osteoblasts was replaced with serum‐free medium and soluble human Fn (30 μg/ml) was added in the presence or absence of BMP‐4 (20 ng/ml). Immunocytochemistry was performed 12 h later using mouse anti‐human Fn antibody, which does not recognize endogenously‐released Fn from osteoblasts. Compared with (A) control, (B) treatment with BMP‐4 markedly enhanced extracellular assembly of Fn. Phase‐contrast images are shown in the left panels. Bar = 10 μm.
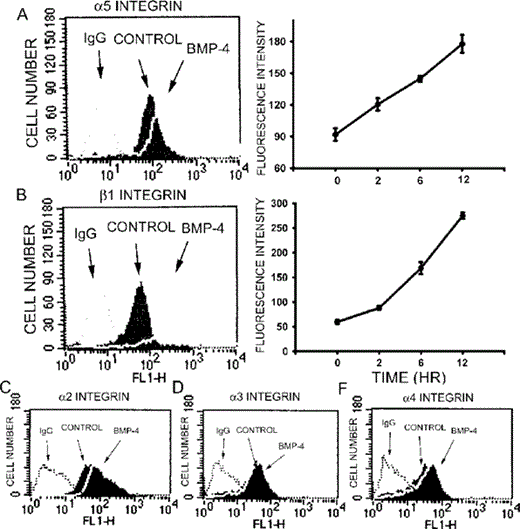
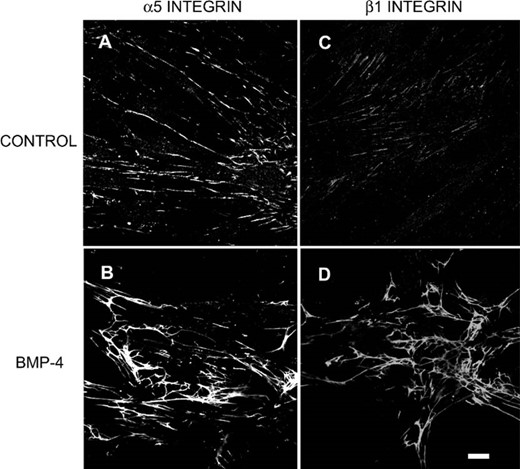
At the cell surface, integrins are intimately involved in constructing fibrillar matrix networks from soluble secreted matrix proteins. We then used flow cytometry to investigate the effect of BMP‐4 on the cell surface expression of integrins. As shown in Fig. 6, incubation with BMP‐4 (20 ng/ml) significantly enhanced the fluorescence intensity of α5 or β1 integrin after 12 h incubation. The cell surface expression of integrins increased in a time‐dependent manner in response to the administration of BMP‐4. In contrast, TGF‐β1 dose‐dependently decreased the surface expression of α5 and β1 integrins after 12 h incubation (α5: the fluorescence intensity was 136.1 ± 19.1, 100.3 ± 13, 86.6 ± 15.1, and 66.3 ± 9.8 for control, TGF‐β1 1 ng/ml, 10 ng/ml, and 100 ng/ml, respectively; β1: the fluorescence intensity was 61.9 ± 3.9, 50.3 ± 1.9, 41.2 ± 2.8, and 29.1 ± 4.3 for control, TGF‐β1 1 ng/ml, 10 ng/ml, and 100 ng/ml, respectively). On the other hand, effects of BMP‐4 on the expression of different subunits of integrin is rather selective. BMP‐4 treatment only slightly increased the fluorescence intensity of surface expression of α2 (control, 79.7 ± 6.2; BMP‐4, 119.5 ± 9.4, n = 4) but not those of α3 (control, 25.3 ± 7.1; BMP‐4, 27.4 ± 8.1, n = 4) and α4 (control, 26.0 ± 5.5; BMP‐4, 31.6 ± 3.0, n = 4). We then used immunocytochemistry to visualize the localization of integrins. Compared with control (Fig. 7A), treatment with BMP‐4 for 12 h greatly enhanced the clustering of α5 integrin (Fig. 7B). The effect of BMP‐4 on the clustering of β1 integrin was also examined. Similar to action on α5 integrin, treatment with BMP‐4 for 12 h also increased the clustering of β1 integrin (Figs. 7C and 7D).
Increase of the cell surface expression of α5 and β1 integrins by BMP‐4 using flow cytometric analysis. Compared with control, treatment with BMP‐4 (20 ng/ml) for 12 h enhanced the fluorescence intensity of (A) α5 and (B) β1integrins, respectively. The quantitative data are shown in the right panels. The cell surface expression of α5 and β1 integrins increased in response to BMP‐4 in a time‐dependent manner. BMP‐4 treatment slightly increased (C) α2, but not (D) α3 or (E) α4 integrin. Data are presented as mean ± SE (n = 3).
Effects of BMP‐4 on the clustering of α5 and β1 integrins. Osteoblast cultures were treated with BMP‐4 (20 ng/ml) for 12 h. Immunocytochemistry was performed and fluorescent images were derived from confocal microscope. Compared with (A and C) control, treatment with BMP‐4 markedly enhanced the clustering of (B) α5 and (D) β1 integrins. Bar = 10 μm.
Effect of BMP‐4 on the adhesion of osteoblasts onto Fn
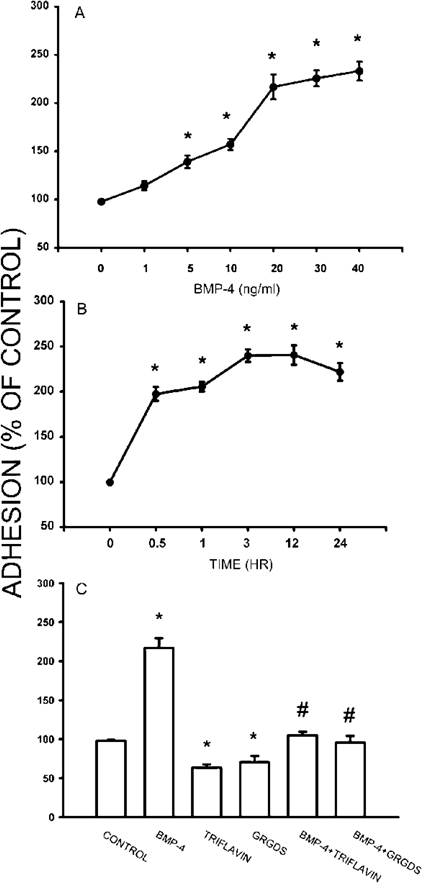
As mentioned above, BMP‐4 enhanced the Fn assembly and the clustering of α5 and β1 integrins. Cell adhesion is dependent on many factors, including the repertoire of ECM proteins and their receptors (e.g., integrins).(25) The assembly of Fn matrix is an important factor to regulate cell adhesion, migration, and differentiation. Cell adhesion was determined by incubating osteoblastic cells with BMP‐4 for 30 minutes before application of cells on Fn‐coated glass for 90 minutes. As shown in Fig. 8A, BMP‐4 increased the adhesion of osteoblasts in a concentration‐dependent manner (5‐40 ng/ml). The number of the adherent cells increased when the BMP‐4 treatment was prolonged, and peak level was reached after 3 h treatment (Fig. 8B). The effect of disintegrins GRGDS peptide and triflavin on the cell adhesion enhanced by BMP‐4 was also evaluated. GRGDS (50 μg/ml) or triflavin (2.8 μM) alone slightly reduced the cell adhesion (Fig. 8C). However, concomitant incubation of GRGDS or triflavin with BMP‐4 (20 ng/ml) markedly inhibited the cell adhesion onto Fn enhanced by BMP‐4 (Fig. 8C). These results suggest that the signaling from RGD‐dependent integrins is involved in the potentiation of BMP‐4 on Fn fibrillogenesis and cell adhesion.
Effect of BMP‐4 on the adhesion of osteoblasts onto Fn. (A) Suspension of osteoblasts was labeled with BCECF/AM and pretreated with different concentrations of BMP‐4 for 30 minutes at 37°. The adherent cells were then examined after incubation for 90 minutes on Fn‐coated 96‐well plates. Note that BMP‐4 potentiates the adhesion of osteoblasts onto Fn in a dose‐dependent manner. (B) Incubation of BMP‐4 (20 ng/ml) with osteoblasts for different time intervals increased the adherent cells time‐dependently. (C) The potentiating effect of BMP‐4 (20 ng/ml, 30‐minute treatment) was inhibited by disintegrins GRGDS and triflavin. Data are presented as mean ± SE (n = 4). *p < 0.05 compared with control. #p < 0.05 compared with BMP‐4‐treated group.
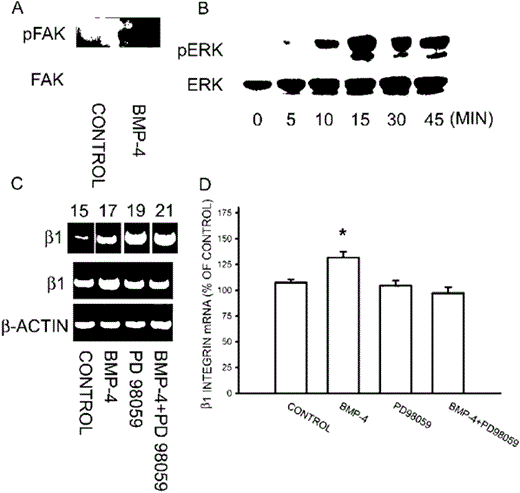
Activation of FAK and ERK by BMP‐4
BMPs have been shown to control bone formation in vivo.(26,27) BMPs signal through heteromeric complexes of transmembrane type I and type II Ser/Thr kinase receptors that then propagate signals to the Smad or other signaling pathway.(28) On the other hand, the signaling through integrins is important in regulating Fn assembly and osteoblast adhesion. Treatment with BMP‐4 for 10‐15 minutes induced phosphorylation of FAK and ERK (Figs. 9A and 9B). We then examined the involvement of ERK activation in the enhancement of Fn fibrillogenesis by BMP. Treatment with MEK inhibitor PD 98059 (30 μM) inhibited the potentiating action of BMP‐4 (20 ng/ml) on the extracellular Fn assembly. The mean fluorescence of Fn underneath the cell was 32.8 ± 2.8, 23.8 ± 2.2, 60.2 ± 3.9, and 37.8 ± 3.2 for control, PD 98059, BMP‐4, and BMP‐4 + PD 98059, respectively. We thus examined the effect of BMP‐4 on the expression of β1 integrin mRNA. As shown in Fig. 9C, amplification was accomplished with 17 cycles, which was within the linear range of PCR product. Treatment of BMP‐4 for 1 h increased the mRNA expression of β1 integrin, which was antagonized by concomitant treatment with PD 98059 (Figs. 9C and 9D). These results suggest that ERK activation may be involved in the action of BMP‐4 on Fn fibrillogenesis.
Activation of FAK and ERK by BMP‐4. (A) Osteoblasts were treated with BMP‐4 (20 ng/ml) for 10 minutes, and FAK was then immunoprecipitated from cell lysate. pFAK was detected by Western blotting. Note that BMP‐4 treatment increased the phosphorylation of FAK. (B) ERK was detected from cell lysate after osteoblasts were incubated with BMP‐4 for different time intervals. Note that BMP‐4 treatment increased the phosphorylation of ERK and reached a peak level at 15 minutes. (C) mRNA level of β1 integrin was measured using RT‐PCR and amplification was accomplished with 17 cycles, which was within the linear range of PCR product (cycles of 15 ∼ 21 were shown in the upper panel). Note that BMP‐4 treatment enhanced the mRNA expression of β1 integrin, which was antagonized by MEK inhibitor PD 98059. The quantitative data were shown in D. (mean ± SE, n = 3). *p < 0.05 compared with control.
DISCUSSION
BMPs belong to a group of proteins that act to induce the differentiation of mesenchymal‐type cells into chondrocytes and osteoblasts before initiating bone formation.(29) The results from this study provide evidence that BMP also regulates Fn fibrillogenesis in cultured rat osteoblasts. The dynamic nature of these Fn fibrils is related to the differentiation and functional regulation of osteoblasts. It has been reported that osteoblasts become increasingly dependent on Fn for survival when they differentiate and form nodules.(30)
Regarding Fn fibril formation in osteoblasts, immunocytochemistry was performed in nonpermeabilized cells to show the extracellular assembly of endogenously released Fn. Here it was found that BMP‐2, BMP‐4, and BMP‐7 enhanced Fn fibril formation. Enhancement of Fn fibrillogenesis may result from the increase of Fn synthesis and/or extracellular Fn assembly. The action mechanism of BMPs was investigated using BMP‐4. To examine extracellular Fn assembly apart from Fn synthesis, soluble human Fn was added into cultured medium, and specific mouse anti‐human Fn monoclonal antibody was used for immunocytochemistry. The results indicate that BMP‐4 markedly potentiates the extracellular assembly of Fn. Western blot showed that the cytosolic protein level of Fn is also upregulated by BMP‐4. Therefore, BMP‐4 may increase both synthesis and extracellular assembly of Fn.
Direct osteoblast interactions with the extracellular matrix are mediated by a selective group of integrin receptors including α5β1, α3β1, αvβ3, and α4β1.(31,32) α5β1 integrin, a specific Fn receptor, mediates critical interactions between osteoblasts and Fn required for both bone morphogenesis and osteoblast differentiation.(22) Interfering with interactions between Fn and integrin Fn receptors in immature fetal rat calvarial osteoblasts suppressed formation of mineralized nodules in vitro and delayed expression of tissue‐specific genes, including osteocalcin.(21,22) Integrin clustering and cell adhesion induce the phosphorylation of FAK.(33) Phosphorylation of regulatory proteins in adhesion plaques, such as talin, paxillin, tensin, integrins, and FAK seems to control the architecture of Fn matrix. Here we also show that BMP‐4 treatment increased FAK phosphorylation. Furthermore, our findings in flow cytometric analysis and immunocytochemistry of α5 and β1 integrins are correlated to the increase of Fn assembly. In addition to the increase of clustering of these integrins, the protein levels and mRNA of α5 and β1 integrins are also upregulated by BMP‐4. To verify the colocalization of α5 and β1 integrins, further experiments are needed using two‐color detection.
Adhesion and cell spreading have been shown to be correlated with the ability of the cell to survive and to initiate proliferation on a substrate, with increased cell spreading associated with increased cell survival and cell cycling.(34,35) RGD peptides are known to disrupt integrin‐dependent cell adhesive events.(36) RGD cell binding site is essential for initiation of extracellular matrix assembly. Treatment of osteoblasts with GRGDS or triflavin markedly inhibited Fn assembly and cell adhesion. These data suggest that RGD‐dependent integrins are involved in Fn fibrillogenesis and cell adhesion of osteoblasts. BMP‐4 seems to modify the expression of integrin receptors and the attachment of cells to Fn.
TGF‐β is a well‐described regulator of the synthesis and degradation of extracellular matrix. BMPs have been included in the TGF‐β superfamily based on the homology of their protein structure to TGF‐β. Despite their structural homology, BMPs and TGF‐β bind to unique receptors. TGF‐β and BMP‐4 have been shown to exhibit similar properties in stimulating collagen synthesis and protein accumulation in osteoblasts.(37) Although BMPs are a group of peptide growth factors closely related to TGF‐β, BMPs and TGF‐β differentially regulate Fn assembly of osteoblasts. Here we found that TGF‐β inhibits, whereas BMPs enhance, extracellular Fn fibrillogenesis. The result is consistent with the finding that TGF‐β has been reported to decrease Fn and osteocalcin mRNA levels, although it increases collagen synthesis.(38,39) BMP and TGF‐β also exert an opposite effect on the expression of ALP in chondrocytes and osteoblasts; BMP increases whereas TGF‐β inhibits ALP activity. In addition, their effects on gene expression and differentiated function of preosteoblasts are also different.(40) It is well recognized that Smad proteins such as Smad1 and Smad5 act as mediators of BMPs while TGF‐β signaling is dependent on Smad 2 and Smad 3.(12,26,28) The effect of BMP and TGF‐β on the expression of integrin is quite complex depending on the types of integrin and cell lines examined.(41) It has been reported that TGF‐β increased α3 and α5 integrins but decreased α1 integrin on chondrocytes.(42) On the other hand, the effects of TGF‐β on the expression of integrins may be different in fetal and adult cells. The expression of α3 and β1 integrin subunits was increased when TGF‐β was added to the medium of adult fibroblast, whereas the levels of the α1 and α2 subunits were unchanged. In contrast, fetal fibroblasts treated with TGF‐β showed a decrease of α1, α2, and β1 integrin expression but no change in α3 integrin.(43) Here we found that TGF‐β decreased the cell surface expression of α5 and β1 integrins in embryonic primary cultured osteoblasts, which are correlated to the inhibition of extracellular Fn fibrillogenesis by TGF‐β. Whether TGF‐β exerts similar regulation on other types of integrin subunit needs further investigation. In addition to Smad pathway, other signaling pathway such as the ERK superfamily has also been shown to be the mediator of BMP signaling.(7) The modulation of integrin signaling by BMPs is not fully understood. Here we found that treatment with BMP‐4 time‐dependently increased ERK phosphorylation. The increase of Fn fibrillogenesis and β1 mRNA expression was antagonized by MEK inhibitor PD98059, indicating that ERK activation may be involved in the regulation of Fn assembly and cell adhesion by BMP‐4.
In conclusion, our results show that BMP‐4 increases Fn fibrillogenesis from both endogenously released or exogenously applied soluble Fn. BMP‐4 upregulates the synthesis and clustering of α5 and β1 integrins. ERK activation is involved in the action of BMP‐4 in the dynamic regulation of Fn and bone tissue remodeling.
Acknowledgements
We thank Dr Tur‐Fu Huang for the kind gift of the disintegrin triflavin. This work was supported by research grants from the National Science Council (NSC 90‐2314‐B002‐193 and NSC 90‐2315‐B002‐013).
REFERENCES
Author notes
The authors have no conflict of interest.