-
PDF
- Split View
-
Views
-
Cite
Cite
Michael D. Brodt, Cara B. Ellis, Matthew J. Silva, Growing C57Bl/6 Mice Increase Whole Bone Mechanical Properties by Increasing Geometric and Material Properties, Journal of Bone and Mineral Research, Volume 14, Issue 12, 1 December 1999, Pages 2159–2166, https://doi.org/10.1359/jbmr.1999.14.12.2159
Close - Share Icon Share
Abstract
In vivo murine models are becoming increasingly important in bone research. To establish baseline data for researchers using these models, we studied the long bones from C57BL/6 female mice, a strain that is widely used in bone research. We determined the femoral structural and material properties in both torsion and four‐point bending for mice at ages 4–24 weeks. Measurements of femoral cross‐sectional geometry and tibial densitometric properties were also obtained. Results indicated that all structural properties (except ultimate energy), changed significantly with age (p < 0.001). Ultimate torque, ultimate moment, torsional rigidity, and bending rigidity all increased to peak values at 20 weeks, whereas ultimate rotation and ultimate displacement decreased to minimum values at 20 weeks. Our data indicate that increases in the material properties contributed more than increases in cross‐sectional geometry to the changes in structural rigidity and ultimate load. For example, from 4–20 weeks torsional rigidity increased 1030%, while shear modulus increased 610% and the polar moment of inertia (a measure of the geometric resistance to rotation) increased by only 85%. Changes in the cross‐sectional geometry with age were due to increases in periosteal diameter and decreases in endosteal diameter. In general, both torsion and bending techniques revealed large changes in structural and material properties with age. We conclude that peak bone strength is not achieved before 20 weeks in C57BL/6 female mice, and that torsion and four‐point bending tests are equally well suited for evaluating mechanical properties of murine long bones.
INTRODUCTION
In vivo murine models are becoming increasingly important in bone research because they allow investigators to study interactions between the metabolic and functional characteristics of bone in animals with targeted genetic alterations. Mechanical testing is often used to evaluate the functional characteristics of bone in mice with altered skeletal metabolism.(1–7) Two issues that must be considered when designing such in vivo studies are age at the time of functional evaluation and the technique for mechanical testing.
Age determines, in part, whether the skeleton is in a state of modeling or remodeling and whether or not skeletal maturity has been attained. In mechanical terms, skeletal maturity can be considered to correspond to the time of peak bone strength. There are no reports characterizing the mechanical properties of long bones from growing mice and identifying when peak bone strength is attained, although such data have been reported for rats.(8–10) Skeletal status at the time of functional evaluation may also determine whether or not treatment and/or genetic effects are significant. Bonadio et al.(3) reported that the failure load of femurs from Mov13 mice were not different from controls at 8 weeks of age, but were different at 15 weeks, and Mikic et al.(6) reported that differences in failure torque for femurs from BMP‐5–deficient mice and controls were not significant at 4 weeks, but were significant at 26 weeks.
Three types of tests are commonly used to evaluate whole bone mechanical properties: three‐point bending,(2,11–14) four‐point bending,(1,3–4,13) and torsion.(1,3–4,6,8–9,12–13,15) One limitation of three‐point bending is that the applied bending moment is not uniform along the length of the bone and thus fracture may not occur at the weakest point.(16) Torsional and four‐point bending tests do not have this limitation and both are considered appropriate for whole bone testing,(17) yet they stress the bone in different ways. A torsion test provides a measure of shear stiffness and strength, whereas a bending test provides a measure of tensile stiffness and strength. Shear and tensile parameters may not be equally sensitive to changes associated with bone growth, although few authors have reported both shear and tensile properties of bone in the same study.
Mechanical properties of bone can be obtained at the structural (whole bone) level and at the material (bone tissue) level. Increases in the structural properties of bones from growing animals have been attributed to changes in both cross‐sectional geometry (“quantity”) and material properties (“quality”).(9,13,18,19) Cross‐sectional geometry may be determined from measurement of the bone before testing or from measurement of the contralateral bone, and structural properties determined from a torsion or bending test. Material properties can then be estimated from the geometric and structural properties using engineering equations.(17)
To establish baseline data for researchers using in vivo murine models, we studied the long bones from C57BL/6 female mice, a strain that is widely used in bone research.(1,3,20,21) We determined the femoral structural and material properties in both torsion and four‐point bending for mice at ages 4–24 weeks. We hypothesized that there would be growth‐related increases in the structural properties of the bones due to both cross‐sectional and material effects. We also hypothesized that torsion and bending tests would be equally sensitive in detecting changes in bone mechanical properties with age.
MATERIALS AND METHODS
Animals
Groups of 15 female C57BL/6 mice were obtained at 4, 8, 12, 16, 20, and 24 weeks of age (Taconic, Germantown, NY, U.S.A.). The mice were euthanized by CO2 hypoxia and weighed. All procedures were approved by our institutional animal studies committee. Femora and tibiae were isolated and cleaned of soft tissue. The length of the right femur was measured from the greater trochanter to the most distal aspect of the femoral condyles using digital calipers (resolution 0.01 mm). Bones were then wrapped in gauze soaked with phosphate‐buffered saline (PBS), placed in a sealed tube, and stored at −20°C.
Cross‐sectional geometry
Right femora were embedded for cross‐sectional analysis following standard procedures for undecalcified bone (Osteo‐Bed Bone Embedding Kit; Polysciences, Warrington, PA, U.S.A.). Sections were cut distal to the third‐trochanter (Isomet; Buehler, Lake Bluff, IL, U.S.A.) and ground to a thickness of 0.1 mm. Sections were then stained using 5% silver nitrate. One section per bone was imaged using a light microscope equipped with a CCD camera. The medial‐lateral and anterior‐posterior diameters (endosteal and periosteal) and average cortical thickness were determined using image processing software (Scion Image; Scion Corp., Frederick, MD, U.S.A.). The FORTRAN program VA‐TWIST(22) was used to calculate the geometric properties of the cortical bone cross‐sections: area, polar moment of inertia, torsional constant (K), geometric maximum shear stress (θnorm), bending moment of inertia (Iap, Iml), and bending radius (ymax). Bending radius was defined as the maximum distance from the centroid of the bone to the anterior periosteal surface.(23) The precision of the technique used to calculate cross‐sectional properties was determined by analyzing five different bone sections three times each. The average coefficient of variation based on these three trials was 1% or less for all cross‐sectional parameters except for the geometric maximum shear stress, which had a coefficient of variation of 4%.
Mechanical testing and structural properties
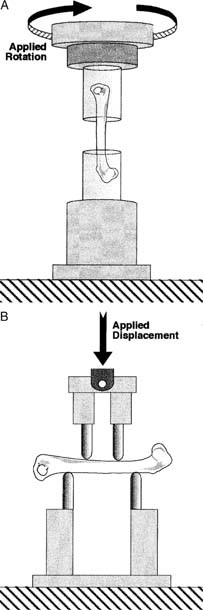
Left femora were mechanically tested to failure, eight in torsion and seven in bending in each group (Fig. 1). Femurs designated for torsion testing were thawed and the ends potted in plastic tubing using polymethylmethacrylate (PMMA). Our potting technique accommodated the increase in femoral length with age and allowed for a uniform region of the diaphysis to be tested for all age groups. The length of the testing, or gage, region was set using custom fixtures that also ensured proper axial alignment. Gage lengths were based on femoral length measurements and were 3.3 mm for the 4 week, 5.5 mm for the 8 week, 6.5 mm for the 12 and 16 week, and 7.0 mm for the 20 and 24 week groups. These gage lengths allowed 3.5–4.0 mm of the distal femur to be potted, while the proximal femur was always potted to the apex of the third trochanter. Bones used for bending tests were not potted. They were placed in the testing fixtures such that a similar diaphyseal region of bone was loaded as in the torsion tests. The distance between the two upper (loading) points was 2.8 mm for the 4 week, 5.0 mm for the 8 week, 6.0 mm for the 12 and 16 week, and 6.5 mm for the 20 and 24 week groups. The distance between the lower (support) points was the upper distance plus 4.0 mm.
(A) Applied rotation on the proximal end of the mouse femur with the distal end held rigidly produced a torque (that is, twisting moment) about the long axis of the bone causing a spiral type fracture. (B) Applied displacement on the posterior side of the bone produced a bending moment in the anterior‐posterior plane, causing the bone to flex and a crack to form on the anterior (tensile) surface of the bone and propagate posteriorly until complete fracture.
Prior to testing, the bones were soaked for 3 h in PBS at 23°C to ensure hydration.(11) Mechanical tests were conducted using a materials testing machine (8500R; Instron, Canton, MA, U.S.A.) fitted with an appropriate sized torque (177 Nmm torque cell, QWLC‐8M; Sensotec, Columbus, OH, U.S.A.) or force (111 N force cell, 3397; Lebow, Troy, MI, U.S.A.) transducer. Torsional specimens were internally rotated about the long axis of the bone at 1°/s until failure.(5) Bending specimens were loaded by a flexion moment in the anterior‐posterior plane(3) at a displacement rate of 0.03 mm/s, which resulted in a similar time to failure as the torsion tests. Torque‐rotation (torsion) or force‐displacement (bending) data were collected using a computerized data acquisition system (Labview 5.0; National Instruments, Austin, TX, U.S.A.).
To allow direct comparison of data from bones of different gage lengths, rotation and displacement were normalized using terms derived from engineering analysis of torsion and four‐point bending.(24) Rotation data were divided by the gage length of the specimen, and all structural parameters were determined from the torque versus normalized rotation curves: ultimate torque (Tult), torsional rigidity (slope of the linear portion of the curve), ultimate rotation, and ultimate torsional energy (area under the curve). Displacement data were divided by the quantity (3aL − 4a2)/6, where L is the distance between the support points, and a is the distance between the loading and support points (2 mm). Structural properties were then determined from the moment (force × a/2) versus normalized displacement curves: ultimate moment (Mult), bending rigidity, ultimate displacement, and ultimate bending energy.
Material properties

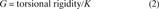

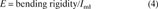
Densitometric properties
Left tibiae were used to determine cortical density and ash fraction. The distal tibia (below the fibular insertion) and proximal tibial plateau were removed and the marrow was then flushed with PBS leaving only cortical bone. Dry weight was determined after drying the bones at 90°C for 12 h, and volume was determined using Archimede's principle.(3) Dry density was defined as dry weight divided by the bone volume. Ash weight was determined after ashing the bones in a muffle furnace at 600°C for 12 h. Ash fraction was calculated as the ash weight divided by dry weight.
Statistical analysis
One‐way analysis of variance (ANOVA) was performed for all measures with age as the factor (StatView 4.0; Abacus Concepts, Berkeley, CA, U.S.A.). To allow comparisons between data from torsion and bending tests, we normalized structural and material properties by the respective mean values at 4 weeks. Two‐way ANOVA was performed on the normalized data with age and testing method as factors. Statistical significance was considered at p < 0.05. Results are presented as mean (SD) values.
RESULTS
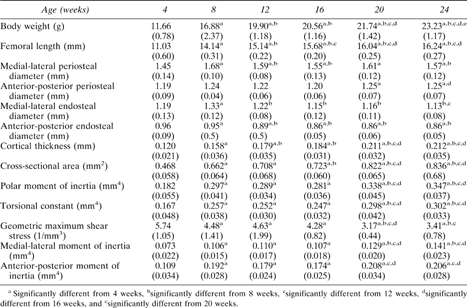
Body weight increased 100% from 4–24 weeks, while femoral length increased 45% from 4–20 weeks (p < 0.001; Table 1). Femoral length did not increase significantly past 20 weeks, in contrast to body weight which continued to increase up to 24 weeks.
Mean (SD) Values of Body Weight, Femoral Length, and Cross‐Sectional Geometric Properties
Mean (SD) Values of Body Weight, Femoral Length, and Cross‐Sectional Geometric Properties
Cross‐sectional geometry
All properties calculated from the femoral cross‐sections changed significantly with age (p < 0.001; Table 1). Average periosteal diameter increased by 7% from 4–24 weeks, while average endosteal diameter decreased by 7%, indicating similar rates of periosteal and endosteal bone formation. Increased bone size was evidenced by increases of 75–90% in cortical thickness, area and moments of inertia from 4–20 weeks. With the exception of the periosteal and endosteal diameters, there were no significant differences in cross‐sectional properties between 20 and 24 weeks.
Structural and material properties
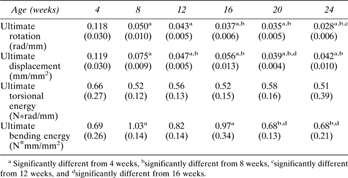
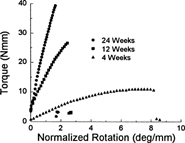
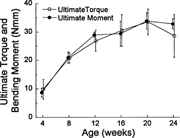
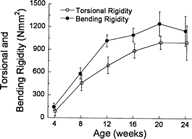
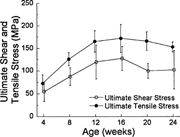
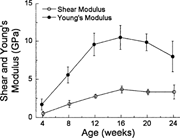
Ultimate load structural rigidity increased with age, while deformation to failure decreased with age (Fig. 2). Ultimate torque and ultimate moment increased by 240% and 290%, respectively, from 4–20 weeks (Fig. 3), while torsional rigidity and bending rigidity increased by 1030% and 770% (Fig. 4). There were no significant changes in ultimate load or rigidity after 20 weeks of age. Ultimate torsional energy did not change with age (Table 2), indicating the increased ultimate torque with age was offset by decreased ultimate rotation. Ultimate shear stress and ultimate bending stress increased by 120% and 125%, respectively, from 4–12 weeks, after which there were no significant differences (Fig. 5). Shear modulus increased 610% from 4–16 weeks, while Young's modulus increased 460% from 4–12 weeks (Fig. 6).
Mean (SD) Values of Ultimate Deformation (Rotation, Displacement) and Energy from Torsion and Bending Tests
Mean (SD) Values of Ultimate Deformation (Rotation, Displacement) and Energy from Torsion and Bending Tests
Representative torque versus normalized rotation curves for femurs at 4, 12, and 24 weeks of age. Ultimate (maximum) torque and rigidity (slope) increased with age, while ultimate rotation decreased. Data from the bending tests demonstrated similar trends.
Ultimate torque and bending moment increased with age to peak values at 20 weeks. There were no important differences between the results obtained using the two testing methods.
Torsional and bending rigidity increased with age to peak values at 20 weeks. Although absolute rigidity values were higher for bending than torsion, the percent increase from 4–20 weeks was higher for torsion. Despite these minor differences, the age‐related trends were not different between torsion and bending.
Ultimate shear and tensile stress increased with age to peak values at 12 weeks. In contrast, ultimate torque and moment reached peak values at 20 weeks, suggesting that bone material strength in C57BL/6 female mice matures before whole bone failure load.
Shear modulus peaked at 16 weeks, while Young's modulus peaked at 12 weeks. By contrast, the corresponding structural properties (torsional and bending rigidity) did not peak until 20 weeks, indicating that bone material stiffness in C57BL/6 female mice matures before whole bone stiffness.
Structural properties normalized to their respective 4 week values depended significantly on testing method. Normalized values of ultimate moment, ultimate displacement, and ultimate bending energy increased more than did ultimate torque, ultimate rotation, and ultimate torsional energy, whereas rigidity increased more in torsion than bending. Testing method was also significant for modulus, because shear modulus (torsion) increased more than Young's modulus (bending). These results indicate that torsional stiffness properties (rigidity and moduli) increased more with age than did bending stiffness properties, whereas bending ultimate properties (load, deformation, energy) increased more with age than did torsional ultimate properties. In other words, with aging the bones got stiffer in torsion than in bending, but ultimately resisted less torsion than bending.
Densitometric properties
Densitometric analysis indicated that dry density (p = 0.97) and ash fraction (p = 0.51) did not change with age (Table 3).
Density and Ash Fraction of the Left Tibia Did Not Change Significantly with Age (p > 0.05)
Density and Ash Fraction of the Left Tibia Did Not Change Significantly with Age (p > 0.05)
DISCUSSION
We mechanically tested the femurs from growing C57BL/6 female mice in torsion and bending and measured cross‐sectional geometry in order to determine both structural (whole bone) and material (bone tissue) properties. We hypothesized that there would be growth‐related increases in structural properties due to increases in both cross‐sectional geometry and material properties. Our data indicate that all structural properties, except ultimate energy, changed significantly with age. Ultimate torque, ultimate moment, torsional rigidity, and bending rigidity increased with age, whereas ultimate rotation and ultimate displacement decreased with age. The changes in structural properties were associated with changes in both cross‐sectional geometry and material properties, although increases in the material properties contributed more than increases in cross‐sectional geometry to the changes in structural rigidity and ultimate load. For example, torsional rigidity increased 1030%, while shear modulus increased 610%, and the polar moment of inertia (a measure of the geometric resistance to rotation) increased by only 85%. These relative differences are in contrast to data from Ekeland et al.(8) and Keller et al.,(9) who reported that cross‐sectional geometry increased more than material properties in rat long bones. Torzilli et al.(13) reported that changes in cross‐sectional geometry and material properties contributed equally to the changes in structural properties of canine long bones. Differences between our findings and those of previous studies may be due to differences in technique or to differences between species.
We observed that densitometric properties remained constant from 4–24 weeks (Table 3), which is consistent with the findings of Bonadio et al.(3) This finding is in contrast, however, to computed tomography data from Beamer et al.(25) which indicate that femoral bone density increases by ∼25% from 8–16 weeks in C57BL/6 females. Although our density data were based on tibiae rather than femora, Simske et al.(26) reported no differences between tibial and femoral bone densities in mice. Regardless of whether our data or Beamer's are considered more representative of the density changes in growing mice, the large increases in bone material properties we observed with aging are likely to reflect changes in the organic phase as well as the mineral phase. While we did not analyze collagen content, Vogel(10) reported that the soluble content of collagen decreases with age in rat bones, probably due to an increase in the number of collagen cross‐links.(18,27) The mechanical properties of bone have been reported to depend strongly on the number of collagen cross‐links.(4,28) Thus, the increases we observed in bone material properties of growing C57BL/6 mice may be due to increases in both bone mineral density and collagen cross‐links, although further investigation is needed to confirm these associations.
Our second hypothesis was that torsion and four‐point bending would be equally sensitive in detecting changes in mechanical properties of femurs from growing mice. In general, both testing techniques revealed large changes in structural and material properties with age. When examined in detail, we determined that resistance to deformation (i.e., rigidity and modulus) increased at a slightly higher rate with age for torsional loading than for bending, whereas resistance to fracture (i.e., ultimate load, stress) increased more for bending than for torsion. Further study is needed to confirm these differences and to determine their functional significance, if any. Despite the subtle differences between testing methods, we conclude that either torsion or four‐point bending is suitable for evaluating whole bone mechanical properties of murine femora. The conclusion is consistent with Ekeland et al.,(8) who determined that there were no important differences between testing methods when applied to rat femora.
Many previous investigators have addressed issues related to testing technique. Burstein and Frankel(16) recommended torsion testing for analysis of whole bone mechanical properties, in part because a testing apparatus for torsion could be more easily constructed and operated than one for bending, and also because geometric effects due to differences in bone length are more pronounced for bending than for torsion. The availability of modern materials testing machines now makes bending tests no more difficult to conduct than torsion tests for many investigators. In addition, geometric effects in a bending test can be accounted for in the manner that we and others(3) have described. Torsion testing neessitates that the ends of the bones be potted for gripping, which introduces several potential problems, including errors in axial alignment and loosening of the bone in the potting material.(15) To address the alignment issue, we utilized a potting fixture that clamped the bone in the middle third of the diaphysis in order to define its axis, thus allowing the bone axis to be aligned with the axis of the potting tubes. Related to the issue of specimen loosening, our PMMA potting technique resulted in no evidence of a nonlinear “toe” region in the torque‐rotation curves, which would indicate bone‐PMMA relative motion. Keller et al.(9) applied strain gages directly to their rat long bones in order to measure deformation and eliminate problems associated with specimen loosening. However, due to the small size of the mouse femur, we believe that application of strain gages is not practical as a routine technique. The primary difficulty we encountered with the bending tests was that axial rotation of the bone often occurred during the initial part of the test, which may have produced unwanted torsion.(29) To minimize unwanted rotation, the loading points were able to pivot freely so that four points of contact were always ensured, and a small (1 N) preload was applied to maintain the initial alignment of the bone in the fixture.(13)
The primary limitation of our study is that material properties were not determined directly but were derived from the structural properties of the left femur and from the cross‐sectional geometry of the paired right femur. The engineering equations used to derive the material properties are based on the assumption that the cross‐sectional geometry does not vary along the length of the bone, which is not true for the mouse femur. Levenston et al.(22) have estimated that assuming a uniform cross‐section introduces a 40% error when estimating the shear modulus of rat femoral bone. Jepsen et al.(30) using bending tests of micromachined cortical bone specimens from 8‐week‐old C57BL/6 female mice, and reported a Young's modulus of approximately 9.8 GPa and an ultimate tensile stress of 185 MPa. Our values at 8 weeks were 5.6 GPa and 125 MPa, which are 43% and 32% less than the values obtained using the more direct approach of Jepsen et al.(30) Mikic et al.(5) used a similar technique to ours in order to estimate shear modulus and strength of femora from 26‐week‐old mice. Their mean value of modulus was ∼50% greater than ours, while their strength was 35% less, despite the fact that differences between the whole bone properties between the studies were small. These conflicting results highlight the difficulties inherent in estimating material property values from whole bone mechanical properties. Nevertheless, while errors may exist in our estimates of absolute material properties, the relative changes with age we report should not be affected, since the limitations of the method should apply uniformly to all age groups.
An important research implication of our findings is that female C57BL/6 mice, a strain widely used in bone research, do not achieve peak structural properties until 20 weeks of age. Material properties appear to reach peak values slightly earlier (12–16 weeks), but increases in cross‐sectional geometry continue up to 20 weeks, leading to the observed increases in structural properties. These data suggest that 20 weeks is a reasonable estimate for the time at which “skeletal maturity” is attained in C57BL/6 female mice. Studies done using mice at younger ages must consider effects due to continued skeletal modeling.
In summary, our findings demonstrate that the structural, material, and cross‐sectional geometric properties of femurs from C57BL/6 female mice change dramatically with growth and development from 4–24 weeks. Data from torsion and four‐point bending tests reflected similar age‐related changes in bone properties, and either method appears to be well suited for evaluating murine long bones. Based on changes in structural properties and cross‐sectional geometry, we conclude that skeletal maturity is not achieved before 20 weeks of age.