Article contents
Mixed-Layer Illite/Smectite Minerals in Tertiary Sandstones and Shales, San Joaquin Basin, California
Published online by Cambridge University Press: 02 April 2024
Abstract
The southern San Joaquin Valley contains more than 7 km of sedimentary fill, largely Miocene and younger in age. Ancient depositional environments ranged from alluvial fans at the basin margins to turbidite fans toward the basin center. Mixed-layer illite/smectite (I/S) dominates the <2-μm fraction of Miocene shales, and kaolinite is abundant in Miocene sandstones. I/S from carbonate-cemented sandstones contains 5–20% more smectite layers than I/S from uncemented sandstones. The timing of cementation correlates with the proportion of smectite layers in the I/S, suggesting that cementation slowed the illitization process. Smectite and I/S with > 80% expandable layers occur at present burial temperatures of 120°-l 40°C in Miocene sandstones and shales. This highly expandable I/S is restricted to areas covered by thick deposits (1000-2500 m) of Pleistocene sediments. Rocks of similar age and at equivalent temperatures, but covered by <900 m of Pleistocene sediments, contain I/S having low expandabilities (<30%).
Microprobe analyses of 16 discrete smectite and smectite-rich I/S clays indicate an average montmorillonite composition of: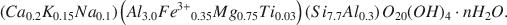
Residence time at different temperatures appears to be an important influence on the percentage of smectite layers in I/S from the San Joaquin basin. Areas containing I/S with high expandabilities (e.g., 95% smectite layers) have a time-temperature index (TTI) of 4.0-4.5 at 120°C, whereas areas containing I/S with low expandabilities (e.g., 30% smectite layers) have a TTI of 5.0. Present data suggest that highly expandable I/S changed to slightly expandable I/S over a narrow temperature interval (10°-20°C). Differences in the potassium availability from detrital components and in the K+/H+ activity ratios of pore water do not appear to be related to the differences in the percentage of smectite layers of these I/S clays.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © 1986, The Clay Minerals Society
References
- 84
- Cited by




