-
PDF
- Split View
-
Views
-
Cite
Cite
Paul W. Harms, Lindsay A. Schmidt, Lauren B. Smith, Duane W. Newton, Maria A. Pletneva, Laura L. Walters, Scott A. Tomlins, Amanda Fisher-Hubbard, Lena M. Napolitano, Pauline K. Park, Mila Blaivas, Joseph Fantone, Jeffrey L. Myers, Jeffrey M. Jentzen, Autopsy Findings in Eight Patients With Fatal H1N1 Influenza, American Journal of Clinical Pathology, Volume 134, Issue 1, July 2010, Pages 27–35, https://doi.org/10.1309/AJCP35KOZSAVNQZW
Close - Share Icon Share
Abstract
A novel H1N1 influenza A virus emerged in April 2009, and rapidly reached pandemic proportions. We report a retrospective observational case study of pathologic findings in 8 patients with fatal novel H1N1 infection at the University of Michigan Health Systems (Ann Arbor) compared with 8 age-, sex-, body mass index–, and treatment-matched control subjects. Diffuse alveolar damage (DAD) in acute and organizing phases affected all patients with influenza and was accompanied by acute bronchopneumonia in 6 patients. Organizing DAD with established fibrosis was present in 1 patient with preexisting granulomatous lung disease. Only 50% of control subjects had DAD. Peripheral pulmonary vascular thrombosis occurred in 5 of 8 patients with influenza and 3 of 8 control subjects. Cytophagocytosis was seen in all influenza-related cases. The autopsy findings in our patients with novel H1N1 influenza resemble other influenza virus infections with the exception of prominent thrombosis and hemophagocytosis. The possibility of hemophagocytic syndrome should be investigated in severely ill patients with H1N1 infection.
Influenza has been recognized as a human illness since the Middle Ages.1 Influenza outbreaks occur in 2 distinct patterns: endemic (seasonal) and pandemic. Pandemics typically occur decades apart and are associated with influenza A virus strains harboring novel forms of the hemagglutinin molecule. Influenza virus subtypes that have been associated with pandemics include H1N1, H2N2, and H2N3. The most severe influenza pandemic recorded occurred in 1918. It was associated with the H1N1 subtype and claimed approximately 50 million lives worldwide.1,2
In early April 2009, the US Centers for Disease Control and Prevention (CDC) reported swine influenza A (H1N1) in 2 children and raised the possibility of human-to-human transmission.3 In June 2009, the World Health Organization confirmed an outbreak of human infections with a novel H1N1 influenza A virus and determined that the outbreak met established criteria for a pandemic.4 The World Health Organization estimates that the novel H1N1 virus spread farther worldwide in 6 weeks than previous pandemic viruses have spread in 6 months.5 As of October 17, 2009, the CDC estimated between 14 million and 34 million cases of novel H1N1 influenza, with between 2,500 and 6,000 deaths.6
The current US case fatality rate for novel H1N1 influenza is estimated to be similar to that for seasonal influenza (0.08%) and lower than the reported case fatality rate for the 1918 pandemic (2.1%). In comparison with seasonal influenza, deaths associated with novel H1N1 infection have disproportionately affected young to middle-aged adults, many of whom lacked known risk factors for serious complications from influenza,2,7–9 a pattern similar to the 1918 influenza pandemic.1 Animal models and clinical observations suggest that, unlike endemic influenza virus that is detected only in the nasal cavity, novel H1N1 localizes to the lower respiratory tree and the nasal cavity, which may contribute to its virulence.9–11
The histopathologic changes associated with novel H1N1 infection in humans have recently been described in only a limited number of studies.12–14 The University of Michigan Health Systems (UMHS; Ann Arbor) received 133 patients with suspected or confirmed novel H1N1 influenza virus infection from May 26 through November 25, 2009, with 16 deaths. Unique clinical findings previously described in novel H1N1 influenza victims at UMHS include obesity, male sex, frequent occurrence of pulmonary thromboemboli, and a high mortality rate among young to middle-aged adults.9 Autopsies were performed in 8 H1N1 deaths. We reviewed findings from these autopsies to further detail the gross and histopathologic features of fatal novel H1N1 influenza virus infection.
Materials and Methods
We reviewed medical records, laboratory data, autopsy reports, gross specimens, gross photographs, and microscopic slides from 8 decedents who died at UMHS from May 26 to December 2, 2009, with novel H1N1 influenza infection. Only patients with positive polymerase chain reaction (PCR) results for influenza A subtype 2009 H1N1 virus were included. None underwent lung biopsy in the course of their hospitalization for H1N1 infection. The time of disease onset was defined as the date on which the onset of respiratory symptoms was reported in patient histories or the date of admission if no symptom onset date was recorded.
Autopsy tissue was collected for microscopic examination from all major thoracic and abdominal organs. For 5 cases, additional lung tissue was collected fresh at autopsy according to published CDC guidelines,15 frozen in liquid nitrogen, and submitted to the CDC, Atlanta, GA, for novel H1N1 PCR and immunohistochemical studies for influenza A by monoclonal antinucleoprotein antibody. Of the 5 cases, 4 were also evaluated by special stains for microorganisms, immunohistochemical studies for common bacterial pathogens (group A Streptococcus, Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae), and bacterial 16s ribosomal DNA (rDNA) PCR. H&E-stained slides of formalin-fixed, paraffin-embedded autopsy tissue were prepared using standard protocols.
Eight control noninfluenza-related autopsy cases from UMHS were selected on the basis of age, male sex, and history of management with extracorporeal membrane oxygenation (ECMO). Autopsy reports, microscopic slides, medical records, and laboratory results were retrospectively reviewed for control cases.
Results
Clinical Features
Eight patients who died with confirmed H1N1 influenza infection at UMHS are the focus of this study. Clinical findings are summarized in Table 1. Eight men had a mean ± SD age of 39.5 ± 14.2 years (range, 23–57 years). Of the 8 patients, 5 were obese (mean BMI, 35.6 kg/m2) and 1 was overweight (BMI, 28.8 kg/m2).16 All patients were admitted to outside institutions with the acute onset of a combination of symptoms that included fever (7), chills (3), cough (4), dyspnea (3), malaise (2), myalgias (2), and diarrhea (2). One patient each also complained of sweats, chest pain, and loss of appetite. Four patients had underlying respiratory illnesses that included obstructive sleep apnea, a combination of obstructive sleep apnea and smoking-related chronic obstructive pulmonary disease, asthma, and sarcoidosis. The patient with sarcoidosis (case 4) had a year-long history of respiratory complaints for which he underwent diagnostic surgical lung biopsy 2 months before hospital admission for an acute illness characterized by fever, malaise, and worsening dyspnea. He was being treated with prednisone for his sarcoidosis at the time. An additional patient (case 3) had a history of non-Hodgkin lymphoma thought to be in remission when he was admitted for a 6-day history of fever, chills, and diarrhea. In all patients, acute respiratory failure developed, requiring mechanical ventilation, before transfer to our facility.
All patients underwent extensive antemortem testing for coinfection with nonviral pathogens that included cultures of blood, bronchoalveolar lavage (BAL) fluid, nasal swabs, urine, and rectal swabs or feces. A blood culture obtained on UMHS hospital day 5, 4 days before death, was positive for Acinetobacter baumannii in case 3. Sputum (hospital day 5) and BAL fluid (hospital days 6 and 7) also grew A baumannii. Cultures of pulmonary secretions were negative in all other patients as were tests for Legionella pneumophila antigen.
In 2 additional patients, blood cultures were positive for S aureus that was methicillin sensitive in one (case 6) and methicillin resistant (MRSA) in another (case 7). In case 6, two positive blood cultures were collected from femoral lines about an hour apart on the first UMHS hospital day, 18 days before the patient died. The first culture grew predominantly coagulase-negative Staphylococcus, and the second grew methicillin-sensitive S aureus. All subsequent blood cultures were negative. In case 7, a blood culture drawn on hospital day 25 grew MRSA, 5 days before death and 20 days after a nasal swab was first positive for MRSA. The patient had an infected tracheostomy site from which cultures obtained on hospital day 26 grew MRSA and vancomycin-resistant Enterococcus faecium and Candida albicans. Cultures from BAL fluid collected on the same date were negative. A BAL fluid culture obtained on hospital day 15 was positive for herpes simplex virus; PCR assays performed on the same sample were negative for influenza A and B and respiratory syncytial virus RNA.
All patients were mechanically ventilated (mean ± SD, 22.5 ± 9.7 days), and 5 required ECMO (mean ± SD, 16.0 ± 10.4 days; range, 5–32 days). The mean ± SD duration of illness from symptom onset (7 patients) or hospitalization (1 patient) to death was 28.5 ± 8.1 days (range, 16–38 days).
Control subjects were mechanically ventilated (mean ± SD, 14.1 ± 8.5 days; range, 1–26 days) and treated with ECMO (mean ± SD, 10.5 ± 7.0 days; range, 1–26 days) for cardiogenic shock (2), sepsis (1), and diffuse lung disease of indeterminate etiology (5). All were men with a mean ± SD age of 39.9 ± 13.3 years (range, 22–61 years). Of the 8 men, 6 were obese (mean BMI, 35.9 kg/m2) and 1 was overweight (BMI, 28.5 kg/m2).
Gross Autopsy Findings
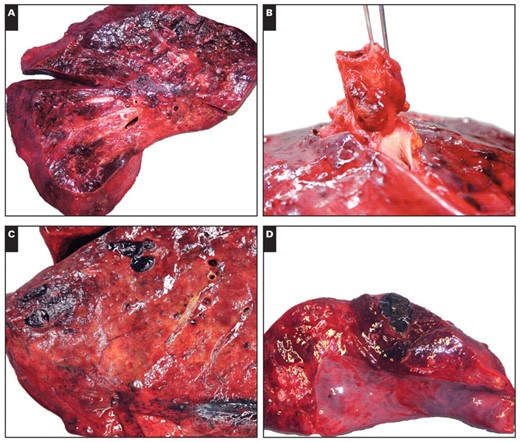
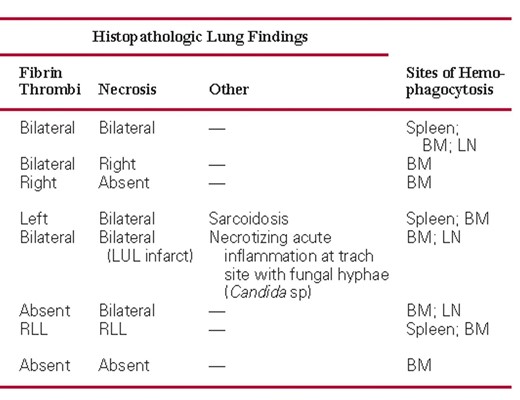
All study cases demonstrated similar macroscopic findings in the lungs. The lungs were heavy, with a mean combined weight of 2,296 g (SD, 377.1 g; range, 1,750–2,690 g) compared with an upper limit of normal of 1,050 g,17 but were not significantly heavier than lungs in control cases (mean combined lung weight, 1,906 g; P = .12). The cut surfaces displayed a firm, glassy, consolidated appearance typical of diffuse alveolar damage (DAD). Prominent central smooth, firm consolidation was present in cases 1 and 3 Image 1A. There was extensive patchy peripheral hemorrhage of lung parenchyma in all but 1 case, resulting in a characteristic hobnail-like, subpleural nodularity with subjacent intraparenchymal hemorrhage in some cases Image 1B. In 5 cases, thrombi were identified in large or small branches of the pulmonary artery in one or both lungs Image 1C. Two patients (cases 2 and 5) had localized hemorrhagic infarcts that in one (case 5) was affiliated with a penetrating chest tube Image 1D. Peribronchial and hilar lymphadenopathy was present in 5 cases (Image 1B). Mucosal necrosis and hyperemia were observed in the large cartilaginous airways in only 3 patients (cases 5, 7, and 8), but histologic evidence of necrotizing tracheobronchitis was identified in 4 patients, including one with an infected tracheostomy site (case 5).
Patient 2 had massive intracranial hemorrhage. Otherwise, only minimal abnormalities were present outside the thorax and were largely confined to the spleen. Splenomegaly was present in 6 patients (cases 1, 2, and 4–7), with a mean ± SD weight in patients with splenomegaly of 465 ± 223 g (range, 280–900 g).
Macroscopic findings in fatalities of novel H1N1 influenza. A, Cut surface of lung with central consolidation and evidence of diffuse alveolar damage. B, External surface of lung with subpleural nodularity and hilar lymphadenopathy. C, Cut surface of lung displaying peripheral pulmonary thrombi. D, Cut surface of lung with focal hemorrhagic infarction.
Microscopic Autopsy Findings
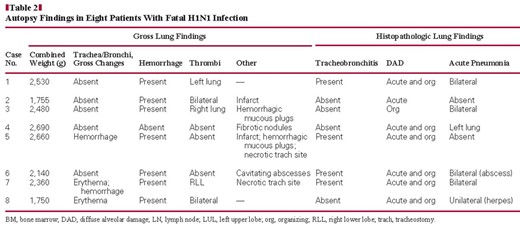
Microscopic findings are summarized in Table 2.
Lungs
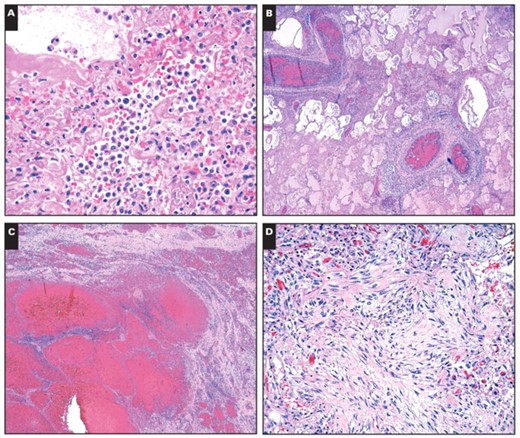
DAD was present in the lungs of all cases with influenza and 50% of control cases Image 2A. In most cases (cases 1 and 4–8), both acute and organizing phases were present, whereas only organizing DAD was present in case 3 and only acute DAD was present in case 2. Alveolar septa were diffusely expanded and distorted by a combination of a mild inflammatory infiltrate in which mononuclear cells predominated, increased numbers of spindled stromal cells consistent with myofibroblasts, and hyperplasia of type II pneumocytes. Associated hyaline membranes were present in most cases. Multiple fibrin thrombi were present in 7 of 8 cases and were accompanied by microscopic zones of infarct-like necrosis in case 5 Image 2B. Small microscopic foci of DAD-associated necrosis were seen in 6 cases. DAD was accompanied by acute diffuse alveolar hemorrhage in case 2 and was characterized by extensive filling of air space with erythrocytes and associated fibrin but without hemosiderin pigment Image 2C. Less prominent hemorrhage was present in cases 1 and 3. Pulmonary hemorrhage was also present in 6 control cases.
Patchy, acute, necrotizing bronchopneumonia was present in 6 cases, comprising a dense air space infiltrate of neutrophils distributed in a distinctly bronchiolocentric manner with associated necrosis. Special stains (Gomorimethenamine silver and Brown-Hopps) were performed on sections from cases 1 and 3 and were negative for bacteria in both, although premorbid cultures were positive for Acinetobacter in case 3.
In case 4, there were chronic fibrotic and acute, poorly organized granulomas within the lung parenchyma, consistent with the patient’s chronic pulmonary disease. Organizing DAD involved the remaining lung parenchyma Image 2D.
Other Organs
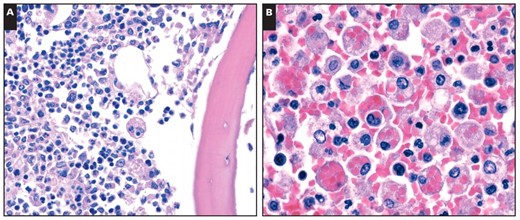
Cytophagocytosis was identified in bone marrow sections obtained from ribs in all cases Image 3A. Especially prominent hemophagocytosis was documented in the spleen Image 3B, lymph nodes, and bone marrow of the patient with the most rapid disease course (16 days from symptom onset to death). Case 3 showed plasmacytosis consistent with recurrent lymphoplasmacytic lymphoma in the bone marrow and spleen. Scattered cytophagocytic cells were seen in 2 control cases, including in 1 patient who died of a possible viral pneumonia.
There was no microscopic evidence of viral encephalitis in any case. Sections of the brain from case 2 displayed multi-focal hemorrhage with diffuse secondary ischemic injury.
There were no specific findings in other organ systems. Extensive postmortem autolysis prevented meaningful analysis of gastrointestinal mucosa.
Postmortem Assays for Viral and Nonviral Pathogens
Postmortem cultures of autopsy lung tissue were performed in 6 cases. Lung culture in 1 case grew A baumannii in the patient with known Acinetobacter infection, as well as C albicans. In another case, postmortem cultures of lung tissue were positive for E faecium and C albicans. Lung cultures from a third patient grew C albicans, rare S aureus, and rare vancomycin-resistant Enterococcus, of uncertain significance. Postmortem lung cultures were negative in the other 3 cases.
Lung tissue from 5 patients was submitted to the CDC for further analysis. PCR for novel H1N1 influenza virus was positive in 3 cases. A fourth case was positive for influenza that was not subtypeable. Immunohistochemical studies for influenza A and bacterial pathogens and bacterial 16S rDNA PCR were negative in all 5 cases.
Discussion
DAD was the most common autopsy finding in our patients. DAD is a relatively nonspecific manifestation of catastrophic acute lung injury typically seen in patients with the adult respiratory distress syndrome regardless of etiology. Pathologic changes associated with seasonal influenza have been extensively studied and are largely confined to the lower respiratory tract where DAD is a common finding in patients with severe disease. In a previous study, hyaline membranes (the histologic hallmark of the early or acute phase of DAD) were present in 2 of 5 surgical lung biopsy specimens reported from patients with sporadic influenza, one of whom died and had organizing DAD at autopsy.18 Hyperemia and extensive hemorrhage were seen in all but one of our cases and have also been observed in previous descriptions of severe disease. Influenza is not associated with any specific cellular alterations (eg, viral inclusions, multinucleation, or cytomegaly). No histopathologic features have been identified that distinguish seasonal from pandemic influenza.1,18–20
Acute bronchopneumonia accompanied DAD in 6 of our cases, including one with the shortest disease interval. This finding suggests that otherwise unexplained DAD and bronchopneumonia in lung biopsy specimens should raise a suspicion of novel H1N1 influenza virus infection. PCR was positive for novel H1N1 influenza virus in our patient with early disease, further suggesting that the combination of DAD and bronchopneumonia may be an early feature of influenza pneumonia. An additional patient with acute bronchopneumonia complicating DAD in our series had a more prolonged course with a secondary bacterial infection as a more likely explanation for this combination of findings.
Pulmonary histopathology in fatalities of novel H1N1 influenza. A, Acute interstitial inflammation accompanied by acute diffuse alveolar damage with hyaline membranes (H&E, ×400). B, Thrombi in pulmonary arteries (H&E, ×40). C, Acute hemorrhage without organization (H&E, ×40). D, Organizing diffuse alveolar damage with fibrosis (H&E, ×200).
Pulmonary thrombi and thromboemboli were conspicuous findings in more than half of our cases. Although microscopic thrombi within the pulmonary arterial system have been described in association with influenza and DAD,1,19 the number, size, and peripheral location of thrombi observed in our study may be unique to novel H1N1 influenza virus infection. We previously reported a high incidence of pulmonary thromboemboli in our population of critical care patients with novel H1N1 influenza.9 Although in vitro and clinical studies have demonstrated evidence that influenza may promote a procoagulant state, a correlation between seasonal influenza infection and pulmonary thromboembolism has not been proven.21
In our cases, several factors were present that may increase risk of pulmonary thromboembolism independent of influenza, such as prolonged immobility and obesity.22,23 The incidence of pulmonary thrombi in our cases (75%) was higher than the reported frequency of pulmonary thromboembolism for intensive care unit patients at autopsy (5%).24 Our frequent observation of peripheral thromboses in the lungs raises the question of whether some thrombi might have formed de novo within the lung vasculature, rather than being embolic from a distant site. Microscopic fibrin thrombi involving small vessels are a common feature of DAD,19 but to our knowledge, in situ thrombosis of larger vessels has not been described in DAD or influenza infection. Notably, we did not find direct evidence of thrombi in organs other than the lungs.
Hemophagocytosis was observed in all of our cases, but none satisfied all criteria for diagnosis of the hemophagocytic lymphohistiocytosis (HLH) syndrome. HLH occurs in primary (familial) and secondary (reactive) forms. The secondary form arises in the setting of strong immune activation, such as severe viral infection, and can be rapidly fatal.25 The diagnosis of HLH requires 5 of 8 clinical and laboratory criteria.25 Two criteria (soluble CD25 levels and natural killer cell activity) were not tested in any of our patients, and an additional three criteria (ferritin, fibrinogen, and triglycerides) were not tested in all cases. The remaining clinical criteria were variably present, with 3 cases meeting 4 criteria, 3 cases meeting 3 criteria, and 2 cases meeting 2 criteria. Given that not all criteria were evaluated, the possibility of HLH cannot be ruled out in 6 cases. HLH has been previously described in influenza infection, including infections caused by avian H5N1 and swine H1N1 influenza virus subtypes.26,27
Hemophagocytosis in deaths of novel H1N1 influenza. A, Cytophagocytosis in bone marrow (H&E, ×600). B, Hemophagocytosis in spleen (H&E, ×400).
Most of our study patients were managed with ECMO during their hospital course. Mixed results regarding recovery from severe H1N1 influenza infection after ECMO management have been reported,9,28–31 with the largest study finding that 71% of patients with severe H1N1 infection survived to intensive care unit discharge after ECMO management.32 Hemorrhagic and thrombotic complications are associated with ECMO therapy.33 In line with this, 1 patient in our series died of intracranial hemorrhage likely secondary to anticoagulation. To our knowledge, no studies have specifically examined the incidence of pulmonary thromboembolism in the setting of ECMO support for pulmonary infection. However, the frequency of pulmonary thromboembolism at autopsy in patients receiving ECMO support for postcardiotomy cardiogenic shock has been reported to be 15.4%,34 which is lower than the rate observed in our study. Although we cannot rule out coagulopathy secondary to ECMO as a contributing factor to the pulmonary hemorrhage and thrombosis observed in H1N1 deaths in this case series, comparison with control cases suggests that these findings were associated with the disease process.
Several recent studies have published pathologic findings at autopsy in novel H1N1 influenza virus infection.12–14 Ours is the first to provide detailed observations regarding findings in patients managed with ECMO. The median age of our patients is similar to that reported by others (28, 34, and 41.5 years).12–14 One study of deaths in New York City described a high rate of obesity (72%) in patients who died in common with our study,12 whereas a study in São Paulo, Brazil, observed a low rate of obesity (2/21 cases).14 Although all of our patients were men, other studies report a roughly equal male/female ratio.12,14 The median number of hospital days in our study (26 days) is higher than that reported in other studies (6 and 7 days).12,14 Mechanical ventilation was an aspect of patient care in all studies. Pulmonary thrombosis was observed in other studies, although at a lower rate than in our patients. Tracheobronchitis and acute bronchopneumonia (with or without evidence of bacterial infection) were common findings across studies. In agreement with our observations, other investigators reported acute DAD in nearly all deaths of H1N1 influenza virus infection. It is interesting that we observed the organizing phase of DAD in the majority of cases (88%), whereas other studies did not report organizing DAD or found it in only a minority of patients.12–14 Two studies reported cytophagocytosis, in agreement with our observations.13,14
Several pathologic parallels exist between the 2009 novel H1N1 influenza and the 1918 pandemic influenza. Our microscopic observations correlate with the extensive reparative and organizing changes reported in resolving influenza infections, for the 1918 pandemic influenza and for influenza in general.1,18,35 In addition, our gross finding of subpleural nodularity consistent with hemorrhagic infarcts was also observed in the lungs of patients who died in the 1918 influenza pandemic.35 A significant component of the pulmonary pathology described for acute cases of the 1918 pandemic influenza is hypothesized to be related to bacterial superinfection. Evidence of bacterial coinfection has been described in 29% of H1N1 influenza-related deaths.36 Culture-proven bacterial superinfection was present in 2 of our cases (25%).
Laboratory assays to detect H1N1 influenza once disease is suspected include direct fluorescent antibody, viral culture, and PCR. PCR allows for subtyping of specific virus strains, including novel H1N1 influenza virus.37–39 Influenza virus was detected by PCR in all 8 of our patients during life. The results of direct fluorescent antibody testing were negative in all cases, consistent with the report that direct antigen testing is less sensitive than PCR for detection of novel H1N1 influenza virus.39 Antemortem viral culture for influenza A yielded mixed results.
Assays for viral and nonviral pathogens in postmortem lung tissue produced varying results. PCR for novel H1N1 influenza virus was positive in the majority of cases tested (60%), including in 1 patient who died 37 days after the onset of symptoms (case 3). The finding that detectable virus may persist in lung tissue for more than a month suggests that PCR analysis of postmortem lung tissue can be useful for confirming novel H1N1 influenza virus infection in patients with a protracted disease course. Culture of lung tissue collected at autopsy proved effective at identifying bacterial pathogens, whereas special stains, immunohistochemical studies, and PCR for bacterial 16S rDNA were negative in all cases. We note that a study released by Gill et al12 after the submission of this manuscript successfully identified influenza virus by immunohistochemical studies in autopsy lung tissue in a majority of cases. This disparity may be due to differences in antibody sensitivity and/or the immunohistochemical protocol.
DAD variably complicated by hemorrhage and acute bronchopneumonia is the most common manifestation of severe infection with novel H1N1 influenza virus. Patients with preexisting diffuse lung disease may be at increased risk for developing pulmonary fibrosis. Hemophagocytosis was a common histologic finding in our small series, and the possibility of hemophagocytic syndrome should be considered in patients with severe H1N1 infection because it may be a contributing factor to the high death rate observed in young patients.
Upon completion of this activity you will be able to:
list the pathologic changes in the respiratory tract associated with fatal H1N1 influenza virus infection.
describe hematopathologic findings in fatal H1N1 influenza virus infection.
compare the utility of laboratory assays commonly used to detect and characterize influenza virus infection.
define basic features of pandemic influenza viruses.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this educational activity for a maximum of 1 AMA PRA Category 1 Credit ™ per article. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Questions appear on p 163. Exam is located at www.ascp.org/ajcpcme.
Supported by the University of Michigan Department of Pathology.
References