An overview of extracellular traps, including NETosis and METosis.
Neutrophil granulocytes play a pivotal role in innate immunity by defending the host against invading pathogens through approaches such as phagocytosis, the formation of reactive oxygen species (ROS), degranulation, and the generation of neutrophil extracellular traps (NETs), a process known as NETosis. NETosis is a type of programmed cell death distinct from apoptosis and necrosis. NETs were firstly described in 2004 as a novel preventive mechanism [5]. Morphological transformations occurring during NETosis consist of the disintegration of nuclear and granule membranes and the combining of nuclear, granular, and cytoplasmic components. A disruption in the plasma membrane incites the release of extracellular chromatin traps. Upon in vitro activation with phorbol myristate acetate (PMA), interleukin 8 (IL-8) or lipopolysaccharide (LPS), neutrophils release granule proteins and chromatin to form NETs through an active process [6]. Thrombosis induced by NETosis is likely the cause of adverse effects of ChAdOx1 nCoV-19 adenoviral vector vaccine against corona virus disease 2019 (COVID-19) in some patients [7].
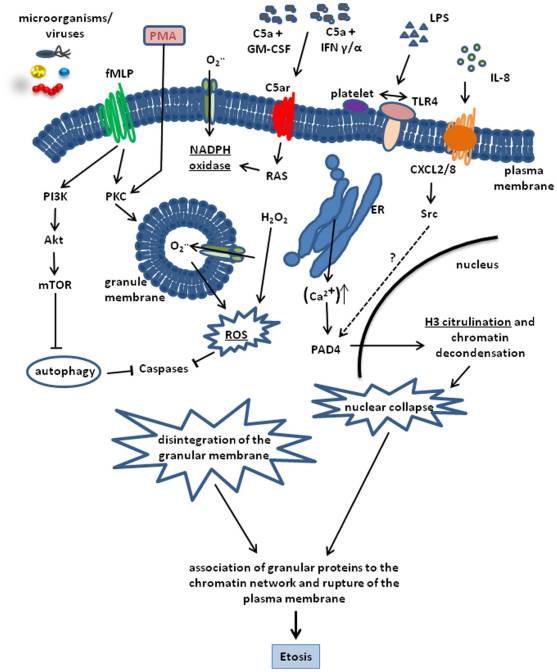
Similarly, other cell types, such as eosinophils, mast cells, and macrophages, can also cause death by this mechanism; therefore, the process was renamed as ETosis, referring to cell death with the release of extracellular traps (ETs). The underlying mechanism behind the ET formation remains mostly unknown, and the biological importance of ETs is just beginning to emerge. This review will focus on the formation and function of ETs by neutrophils and monocytes / macrophages. We will also discuss the mechanisms that regulate the release of ETs during infection and other disease processes. Figure 1 presents schematically the cellular processes involved in the formation of ETs [1]. Table 1 lists the proteins commonly studied in extracellular traps and most cited antibodies against them among the over 60,000 formal publications Labome has surveyed for Validated Antibody Database.
| Protein | Detail | Top three suppliers |
|---|---|---|
| ELANE | elastase | Dako M0752 (13), Santa Cruz Biotechnology sc-53388 (1), Invitrogen MA1-10608 (1) |
| FCGR2A | Fc fragment of IgG receptor IIa | BioLegend 303202 (10), BD Biosciences 557333 (8), Bio-Rad MCA1075 (6) |
| LRP1 | LDL receptor related protein 1 | Abcam ab92544 (10), Invitrogen 37-7600 (3), MilliporeSigma L2420 (2) |
| MPO | myeloperoxidase | Invitrogen MA1-20074 (10), Abcam ab25989 (8), Dako F071401-1 (3) |
| NOX3 | NADPH oxidase 3 | ProSci 7925 (1) |
| NOX4 | NADPH oxidase 4 | Abcam ab133303 (16), ProSci 7927 (2), Invitrogen MA5-32090 (1) |
| PADI4 | peptidyl arginine deiminase 4 | Abcam ab128086 (8), BioLegend 684202 (1) |
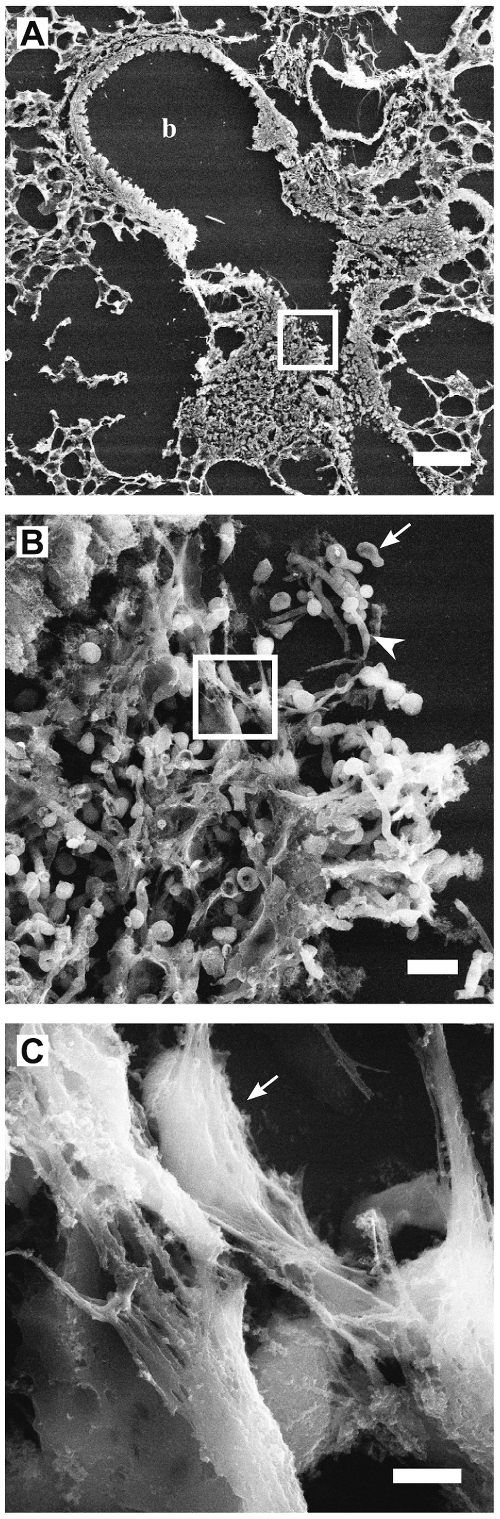
NETosis is neutrophil-related cell death characterized by the secretion of large web-like structures described as NETs. NETs are composed of chromatin fibers with diameters of 15–17 nm that are made up of DNA and histones H1, H2A, H2B, H3, and H4. In addition, NETs consist of proteins from azurophilic granules (i.e., neutrophil elastase (NE) cathepsin G, and myeloperoxidase (MPO)), specific granules (lactoferrin) and tertiary granules (gelatinase) [5]. Figure 2 presents scanning electron microscope images of NETs in an infected mouse lung model [2].
NE is a serine protease that eliminates bacteria [8], and MPO activates the oxidation of halides by hydrogen peroxide [9]. NE and MPO knockout mice are predisposed to bacterial and fungal infections [10]. NE plays a critical role in instigating NET formation and interacts with MPO to initiate chromatin decondensation [11]. Histones are the most abundant NET constituent, and several histones have shown to possess the antimicrobial potential [2]. In particular, the histones H2A and H2B have demonstrated antimicrobial activities against Gram-positive and Gram-negative bacteria and fungi [12].
NETs generation is an active and distinctive process from neutrophil apoptosis and necrosis [3]. NETosis mainly involves reactive oxygen and nitrogen species (ROS/RNS) and activation requires nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and MPO [3, 13]. NADPH-oxidase induces superoxide radicals, prompting the generation of hydrogen peroxide, which is employed by neutrophils to generate hypochlorite that kills bacteria and might further cause lipid peroxidation and membrane damage [14].
There is an accumulating scientific body of evidence that NADPH-mediated ROS formation is essential to drive the NETs formation. For instance, neutrophils derived from patients with chronic granulomatous disease with mutations in the NADPH oxidase that disrupted ROS generation [15] were not capable of inducing the formation of NETs [3]. Besides, ROS scavengers (i.e., N-acetylcysteine, diphenyleneiodonium, or Trolox) prevented NETosis [3] in human neutrophils upon stimulation with PMA and have confirmed NADPH oxidase-dependent NET formation. The exact role of ROS in NETosis formation has not been fully understood, but it is believed to work through two separate mechanisms. Some studies suggest ROS promote the morphological changes noted during NETosis [16]. ROS may alternatively inactivate caspases, prevent apoptosis and advance autophagy. This action leads to the dissolution of cellular membranes [17]. Scientific evidence as to whether there are pathways of NETosis that employ a ROS-independent pathway appears insufficient and contradictory [18].
| NETosis | Apoptosis | Necrosis |
|---|---|---|
| Programmed cell death | Programmed cell death | Cell damage releasing intracellular contents and leading to death |
| Nuclear chromatin decondensation with disintegration of the nuclear membrane into numerous small vesicles | Nuclear chromatin condensation without disintegration of the nuclear membrane | Cellular swelling and bursting |
| Vacuolization | Membrane blebbing | Membrane and organelle disintegration |
NETosis is a new type of cell death, with distinctive features from apoptosis and necrosis. NETosis appears to be a pathway not mediated by caspases and kinases (e.g., RIP-1) since neither inhibition of caspases by zVAD-fmk nor inhibition of RIP1 kinases by necrostatin-1 impacted NETosis. Moreover, the process does not seem to involve DNA fragmentation or phosphatidylserine (PS) exposure of the cellular membrane [2, 17]. However, in NETosis, both the nuclear as well as the granular membranes are subject to fragmentation. Table 2 lists the morphological features of NETosis in comparison with apoptosis and necrosis.
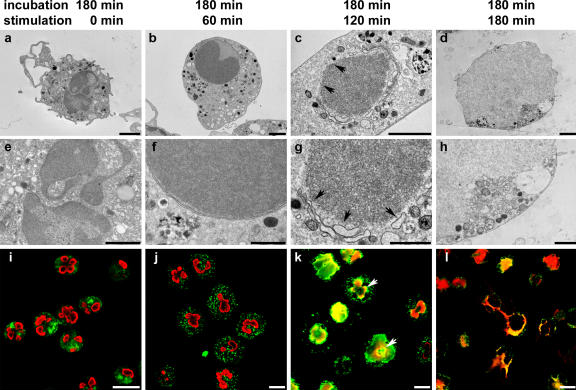
Morphological transformations occurring during NETosis consist of the disintegration of nuclear and granular membranes and integration of nuclear, granular, and cytoplasmic components. Lastly, disruption of the plasma membrane occurs, and DNA combined with the granular substances is secreted into the extracellular environment [3]. Therefore, NETosis should be regarded as a distinct cell death program and is different from both apoptosis and necrosis. Figure 3 shows transmission electron microscopy and confocal immunofluorescence images of the disintegration of the nucleus and granules during NETosis [3].
Two major NET release mechanisms have been reported, suicidal and vital NETosis.
Suicidal NETosis involves the triggering of the Raf-MEK-ERK pathway, NADPH oxidase-dependent pathways, the generation of ROS, and receptor-interacting protein kinase/mixed lineage kinase domain-like-mediated signals [19, 20]. This initiating effect appears to be the primary path for NET release and is an irreversible process. Different receptors, such as Toll-like receptors (TLRs), Fc receptors, or complement receptors, have been reported to be involved in NETosis [5]. Briefly, receptor activation leads to endoplasmic reticulum calcium store release and increased cytoplasmic calcium. Elevated calcium ions stimulate the activity of protein kinase C (PKC), the phosphorylation of gp91phox, and the functional assembly of cytosolic and membrane-bound subunits of NADPH oxidase, leading to the formation of ROS. Under the activation of ROS, the granules, and the nuclear envelope break. Subsequently, the secreted nuclear, granular, and cytoplasmic substances combine (Figure 1).
In this pathway, peptidylarginine deiminase 4 (PAD4)-dependent citrullination of histones promotes decondensation of DNA generating a mixture of DNA and bactericidal proteins, encompassing MPO and NE, which are initially found in intracytoplasmic granules [21]. Afterward, these materials are released from the ruptured plasma membrane. The prerequisite of PAD4 in NET formation is still under exploration. It was reported that PAD4-deficient mice presented decreased NET formation during group A Streptococcus pyogenes infection [21], but they maintained the potential to generate NETs against influenza infection [22].
The majority of the mechanistic understanding on lytic NETosis comes from in vitro studies examining NETosis by treating isolated neutrophils with PMA, for example, [23, 24]. NET secretion by cell death is a slow process (120-240 min) and may allow a window of time for microbes to cause an infection. In contrast, an alternative rapid process (5-60 min) for NET formation has been reported called vital NETosis. This latter process is ROS-independent in response to Staphylococcus aureus [25] and Candida albicans [26, 27]. Moreover, vital NETosis entails vesicular movement of DNA from within the nucleus to the extracellular space [25]. As a result, this pathway preserves the integrity of the plasma membranes, and it does not involve lytic death of the neutrophils [28].
| Method | Detection marker | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Immunostaining and microscopy | Co-localization of neutrophil-derived proteins and extracellular DNA | Easy to perform | Artificial NET staining | [3, 6, 11, 29, 30] |
| Citrullinated histones | Easy to perform | Only PAD4-dependent NETosis measured | [31, 32] | |
| Flow cytometry | MPO-DNA complex; MPO and citrullinated histones | High specificity | Prevalence needed | [33, 34] |
| ELISA | MPO/NE-DNA complex; PAD4/DNA-complex detection | High specificity | Standardization required | [35, 36] |
| Picogreen kit | Cell-free DNA | Available kit | Other cell death-derived DNA can be measured | [23, 32] |
Several methods are developed to analyze NETs [37] including transmission electron microscopy, scanning electron microscopy, and immunofluorescence [25, 27]. During NETosis, the nuclei of neutrophils lose its shape, and the nuclear envelope and the granule membranes disintegrate leading to the mixing of the NET components [6, 38]. Table 3 compares different methods for measuring NETosis.
Co-localization of extracellular DNA and neutrophil-derived proteins, including MPO and NE, was noted by microscopic observation [32, 39]. Enzyme-linked immunosorbent assay (ELISA) was also used to detect the complexes of DNA and neutrophil-derived proteins, including MPO [32], NE, and PAD4 from different patients [29, 35, 40].
Fluorescence-activated cell sorting (FACS) was used to determine NETosis by the detection of MPO and citrullinated histones [33]. SYTOX® Green is a fluorescent dye that is used to label DNA, but it does not permeate the plasma membrane [32]. SYTOX Green showed co-localization of MPO and plasma membrane-appendant DNA of the PMA-treated neutrophils [34]. Cell-free DNA (quantitatively using a kit, Picogreen) was used to detect soluble NET remnants in fluid samples, such as in sera [32, 41].
The above approaches are often combined to address the NET formation. Ali RA et al combined the SYTOX Green assay, the NET-associated myeloperoxidase (MPO) assay, neutrophil elastase staining, and citrullinated histone H3 staining to investigate the effect of antiphospholipid antibodies on NET and its alleviation by adenosine receptor agonism [42]. A Constantinescu-Bercu et al visualize NETosis through co-labeling of neutrophils with cell permeable Hoechst dye and cell impermeable Sytox Green [31], an approach similarly used by LM Silva et al [43].
NETs present broad-ranging potency against a diversity of pathogens including Gram-positive and Gram-negative bacteria, fungi, parasites, and viruses. Furthermore, intrinsic mediators such as hydrogen peroxide [3], cytokines [44], chemokines [45], cholesterol [46] and autoantibodies [29] promote the NET generation. Pro-inflammatory factors, including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-17 and IL-8 [47], can trigger NETosis.
PMA is the most commonly employed stimulus for activating NETosis [31]. However, it is not physiologically applicable, since it does not activate physiological processes in vivo [5]. Several other inducers have been presented, but their NETosis potential has not been consistent. For example, some studies, NETosis was observed after 30 min in the presence of 100 ng/ml LPS [48, 49], whereas in other studies, 10 µg/ml LPS failed to ead to NETosis formation [17]. The differences in experimental setting, timing, and dosing might contribute to such variability.
| Gram–positive bacteria | Gram–negative bacteria | Fungi | Parasites | Viruses |
|---|---|---|---|---|
| Staphylococcus aureus [3, 25, 50] Streptococcus pneumoniae [51, 52] Streptococcus pyogenes [53] | Escherichia coli [54] | Candida albicans [2, 26] Aspergillus fumigatus [55, 56] | Plasmodium falciparum [57] | Feline leukemia virus [58] Human immunodeficiency virus-1 [59] Influenza [22] |
NET formation is limited to particular microbes. The characteristics that determine effective NET stimulants are not well defined. Several bacteria and fungi were reported to potently induce NET formation, such as Staphylococcus aureus [3, 25, 50], Streptococcus pneumoniae [51, 52], Streptococcus pyogenes [53], Escherichia coli [54], Candida albicans [2, 26], and Aspergillus fumigatus [55, 56]. Table 4 outlines microbial inducers of NETs.
Several elements of NETs are instrumental in microbicidal activity. The enzymatic activity of MPO on NETs is a requisite to kill Staphylococcus aureus [18] and the enzymatic activity of NE is essential to generate NETs in a pulmonary model of Klebsiella pneumoniae infection [11]. Antibodies against histones can abolish the NET-moderated microbicidal activity [5]. The fungicidal activity of NETs has been attributed to calprotectin (a complex of S100A8/S100A9), which chelates divalent metal ions. Calprotectin plays a part in the NETs-associated antifungal activity against Candida albicans in vivo infection models [2]. Interestingly, calprotectin is released during Covid-19 infection and its plasma level was found to distinguish between mild and severe forms of COVID-19 [60].
However, some studies challenge the microbicidal capacity of NETs since NET formation appears to be induced by particular pathogens and experimental conditions. For instance, in the presence of DNase treatment in the medium which dismantles NETs, viable Staphylococcus aureus and Candida albicans blastospores were secreted from NETs [61].
Notably, several bacteria can degenerate NETs by nucleases and get away from NET-mediated entrapment and elimination. These involve the Gram-negative pathogen Vibrium cholera [62] and the Gram-positive bacteria Streptococcus pneumoniae [51], Group A Streptococcus [53], Staphylococcus aureus [50], and Streptococcus agalactiae [63]. This degeneration highlights the significant role nucleases play as pathogenic factors.
In the presence of two extracellular nucleases Dns and Xds, Vibrium cholera could break down the DNA component of the NETs [62] and nuclease formation by Staphylococcus aureus promoted resistance against NET-associated antimicrobial activity of neutrophils and lead to lung disease pathogenesis in vivo [50].
Overall, NETs have been shown to kill or inhibit the growth of several bacterial, fungal, and parasite species. However, the general importance of NET-mediated killing of microbes may vary depending on the type of pathogen and the techniques employed to assess the microbial killing.
| Pathophysiological condition | Reference |
|---|---|
| COVID-19 | [64] |
| Gallstone formation | [65] |
| Thrombosis | [66, 67] |
| Systemic lupus erythematosus | [30] |
| Rheumatoid arthritis | [35, 44] |
| Antiphospholipid antibody syndrome | [66, 67] |
| Atherosclerosis | [46] |
| Vasculitis | [29] |
| Pancreatitis | [68] |
| Type I and type II diabetes | [36, 69] |
| Pre-eclamsia | [70] |
| Cardiovascular | [24, 71] |
| Sepsis | [28] |
| Malaria | [57] |
The role of NETosis is unclear between antimicrobial defense and host tissue damage. Several components are toxic to the host cell and trigger autoimmunity. NETs have been implicated in thrombosis [66, 67, 72, 73] as well as diseases like systemic lupus erythematosus (SLE) [30], rheumatoid arthritis [35, 44], antiphospholipid antibody syndrome [66], vasculitis [29], pancreatitis [68], type I [36], and type II diabetes [69], pre-eclamsia [70], cardiovascular problems [71], sepsis [28], and malaria [57]. Table 5 summarizes the pathophysiological conditions in which NETs have been formed.
For example, NETs are elevated in rheumatoid arthritis [16], antiphospholipid antibody syndrome [66], vasculitis [29, 74], SLE [30] and diabetic [75] patients compared to healthy control groups. Neutrophils from rheumatoid arthritis patients demonstrated elevated spontaneous NET production in vitro, accompanied by increased ROS generation, augmented NE and MPO expression, nuclear translocation of PAD4, and modified nuclear morphology [35]. Besides, NET formation promoted the autoimmune response against neutrophil components in individuals with small-vessel vasculitis [74, 76], rheumatoid arthritis [35] and SLE patients [30]. Therefore, inhibiting or cleaving NETs may improve some of the disorders mentioned above.
Also, NETs were shown to be important for thrombosis in murine models of both antiphospholipid antibody syndrome and deep vein thrombosis [66, 67, 73] and NETs elicit pancreatitis by ductal occlusion [68]. Furthermore, secretion of NETs in the vascular space elicited a pro-coagulant condition and triggered activation of platelets causing thrombosis [77].
Aberrant production of NETs and scarcity of DNases to dismantle NETs might contribute to tissue damage and autoimmune diseases in patients [74]. Therefore, the timely removal of NETs may be essential for tissue homeostasis to block the presentation of self-antigens.
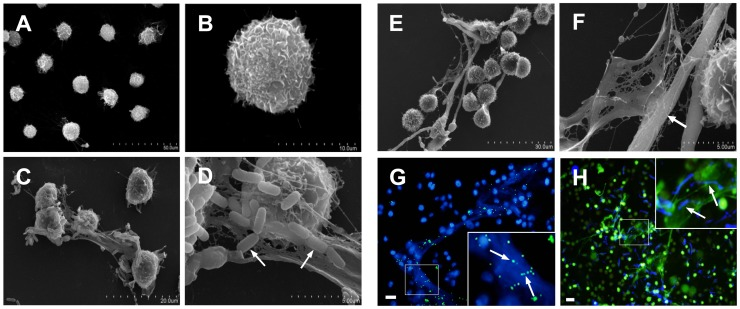
NETosis was first described in neutrophils, but other cell types including monocytes and macrophages are capable of releasing ETs composed of DNA and antimicrobial proteins.
Monocytes/macrophages have been shown to release ETs in a process called METosis [4, 39, 78-80]. Figure 4 presents scanning electron microscope images of METs induced by Escherichia coli and Candida albicans. Treatment with DNase I or nucleases degrade METs. Studies staining well-characterized structures of ETs confirmed the METs features.
| Cell Type | MET components | Reference |
|---|---|---|
| Human alveolar macrophages | matrix metalloproteinase 12 | [81] |
| Human glomerular macrophages | Myeloperoxidase | [82] |
| Human monocyte-derived macrophages | histone H4 | [83, 84] |
| Human peripheral-blood monocytes | histones H2 and H3; elastase; myeloperoxidase; | [85, 86] |
| Mouse macrophages (RAW 264.7 cells) | histones H2 and H4 | [78, 79] |
| Mouse monocyte; macrophage (J774A.1 cells) | histone H2; myeloperoxidase | [4, 79] |
| Rat macrophages | histone H2; myeloperoxidase | [87] |
| Bovine monocytes | histone H3; myeloperoxidase | [88] |
NE has been found in the METs of human peripheral-blood monocytes [89]. In the same way as NETs, MPO has been shown to be part of METs of various macrophages including human glomerular macrophages, human peripheral-blood monocytes, THP-1 macrophage-like cells, murine J774A.1 macrophage-like cells, bovine monocytes, and caprine monocytes [4, 82, 88-90]. Table 6 lists types of monocytes and macrophages reported to generate METs and the protein components associated with METosis.
Macrophage ET generation has been reported to be enhanced by statins, which are inhibitors of the rate-limiting enzyme within the cholesterol biosynthesis 3-hydroxy 3-methyglutaryl coenzyme A (HMG-CoA) reductase. Furthermore, the rise in the formation of METs was noted after preventing the activity of HMG-CoA reductase using small interfering Ribonucleic Acid (siRNA) or after treatment of macrophages with the downstream HMG-CoA reductase product mevalonate [78]. However, the extent to which the molecular mechanism leading to the formation of METs is similar to the process already attributed to NETs remains to be further clarified.
The limited data available shows that METosis is a cell death pathway involving NADPH oxidase dependency, similar to the process in neutrophils. Traditional features of ETs were corroborated in METosis by the co-localization of extracellular DNA with histones H3 or MPO in parasite-entrapping structures. Monocyte-derived ETs were eliminated by DNase I treatment and notably decreased by inhibitors of MPO and NADPH oxidase [88]. Also, treatment of caprine monocyte ET structures with NADPH oxidase inhibitor diphenylene iodondium (DPI) remarkably decreased Etosis [90]. These findings provide evidence for the essential role of ROS and MPO in METosis formation. Treatment of alveolar macrophages with the ROS inhibitor apocinin prevented MET release [81]. It was also shown using the fluorescent dihydrorhodamine 123 that human alveolar macrophages forming METs had a 2-fold increase in ROS fluorescence compared to cells not forming METs [81].
| Gram–positive bacteria | Gram–negative bacteria | Fungi | Parasites |
|---|---|---|---|
| Staphylococcus aureus [78, 80] Streptococcus agalactiae [79] | Klebsiella pneumoniae [85] Mannheimia haemolytica [91] Escherichia coli [89, 92] | Candida albicans [4, 89] | Toxoplasma gondii [93] Besnoitia besnoiti [88] |
Several studies have shown that METs have microbicidal activity against different microorganisms. Several bacteria and fungi were reported to potently induce MET formation, such as Staphylococcus aureus [78, 80], Streptococcus agalactiae [79], Haemophilus influenzae [81], Klebsiella pneumoniae [85], Mannheimia haemolytica [91], Escherichia coli [4, 89] and Candida albicans [4, 89]. Microbial inducers of METs are outlined in Table 7.
ETs display broad-ranging effectiveness against a diversity of pathogens including Gram-positive and Gram-negative bacteria, fungi, parasites, and viruses. Nevertheless, experimental data indicate that ET formation appear to be limited to particular microbes and the characteristics that define effective ET stimulants are not well defined. NETosis especially seems to be strictly governed, and dysregulation has been associated with severe autoimmune conditions. Therefore, as the biological importance of ETs is beginning to be unraveled, the molecular mechanisms regulating the ETs formation and the downstream pathways need to be further explored. Future work characterizing ET properties associated with particular disease states such as kidney transplant rejection [94], or microbial infections will advance the understanding of the role of ETs in diseases and open potential avenues for therapeutic modulation.
- Fuchs T, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231-41 pubmed
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-5 pubmed
- Weinrauch Y, Drujan D, Shapiro S, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002;417:91-4 pubmed
- Nauseef W. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88-102 pubmed
- Belaaouaj A, Kim K, Shapiro S. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185-8 pubmed
- Li G, Mine Y, Hincke M, Nys Y. Isolation and characterization of antimicrobial proteins and peptide from chicken liver. J Pept Sci. 2007;13:368-78 pubmed
- Bartholeyns J, Baudhuin P. [Proceedings: Cytostatic properties of pancreatic ribonuclease A dimer]. Arch Int Physiol Biochim. 1976;84:139-40 pubmed
- Pattison D, Davies M. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem. 2006;13:3271-90 pubmed
- Clark F, Klebanoff S. Chronic granulomatous disease: studies of a family with impaired neutrophil chemotactic, metabolic and bactericidal function. Am J Med. 1978;65:941-8 pubmed
- Clark S, Ma A, Tavener S, McDonald B, Goodarzi Z, Kelly M, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463-9 pubmed
- Nakazawa D, Tomaru U, Suzuki A, Masuda S, Hasegawa R, Kobayashi T, et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasc. Arthritis Rheum. 2012;64:3779-87 pubmed publisher
- Zhang S, Lu X, Shu X, Tian X, Yang H, Yang W, et al. Elevated plasma cfDNA may be associated with active lupus nephritis and partially attributed to abnormal regulation of neutrophil extracellular traps (NETs) in patients with systemic lupus erythematosus. Intern Med. 2014;53:2763-71 pubmed
- Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401-7 pubmed
- Buchanan J, Simpson A, Aziz R, Liu G, Kristian S, Kotb M, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396-400 pubmed
- Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6:e1000873 pubmed publisher
- Yalavarthi S, Gould T, Rao A, Mazza L, Morris A, Nuñez Alvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67:2990-3003 pubmed publisher
- Gupta A, Hasler P, Gebhardt S, Holzgreve W, Hahn S. Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: a link with elevated levels of cell-free DNA?. Ann N Y Acad Sci. 2006;1075:118-22 pubmed
- Hortin G, Gibson B, Fok K. Alpha 2-antiplasmin's carboxy-terminal lysine residue is a major site of interaction with plasmin. Biochem Biophys Res Commun. 1988;155:591-6 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
