Oxidation of Few Substituted Phenols and Reaction Mechanism by Heterocyclic Pyrazinium Chlorochromate and its Biological Activity
1Department of Mechanical Engineering, SRM TRP Engineering College, Irungalur (PO), Tiruchirappalli, Tamilnadu, India.
2Department of Chemistry, Nehru Memorial College (Autonomous), Puthanampatti, / Affiliated to Bharathidasan University, Tiruchirappalli, Tamilnadu, India.
Corresponding Author E-mail: ilavenil@nmc.ac.in
DOI : http://dx.doi.org/10.13005/ojc/390119
Article Received on : 26 Nov 2022
Article Accepted on : 19 Jan 2023
Article Published : 10 Feb 2023
Reviewed by: Dr. Nabeel alradha
Second Review by: Dr. Pooja Sethi
Final Approval by: Dr Amrita Chatterjee
The current investigation focuses on the oxidation of phenol (Phenylic acid) and a few mono substituted phenols in a liquified ethanoic acid medium with pyrazinium chorochromate (PzCC) support. The reaction showed a first kinetic model in PzCC but a fractional order in phenols. The low dielectric constant of the process, which is helped by hydrogen ions, promotes the reaction. The reaction's speed is unaffected by an increase in ionic strength. Meta-substituted phenols, which rely on the field effect for oxidation, are more susceptible to the delocalized effect than para-substituted phenols. The positive proportionality constant (ρ) indicates the presence of an electron deficit reaction site in the slow stage. Non-linearity in the Hammett plot was seen for all of the substituted phenols. An efficient mechanism and rate law were proposed following the computation of the activation and thermodynamic variables. Several gram positive and gram negative, including Entrococcus, Proteus vulgaris, Mycobacterium tuberculosis, and fungi, including A. niger, Fusarium, and Trichoderma, were examined for the antibacterial activity of the compound PzCC using the agar well diffusion method.
KEYWORDS:Antibacterial activity; Hammett Plot; Pyrazinium Chlorochromate; Proportionality Constant; Phenols; Rate Law;
Download this article as:| Copy the following to cite this article: Senthilkumar V, Ilavenil K. K. Oxidation of Few Substituted Phenols and Reaction Mechanism by Heterocyclic Pyrazinium Chlorochromate and its Biological Activity. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Senthilkumar V, Ilavenil K. K. Oxidation of Few Substituted Phenols and Reaction Mechanism by Heterocyclic Pyrazinium Chlorochromate and its Biological Activity. Orient J Chem 2023;39(1). Available from: https://bit.ly/3K0qFKH |
Introduction
Herbicides, medications, and nylon are all produced using phenylic acid (also known as phenol) and its derivatives. Phenylic acid is proven to damage the environment and kill out living things at low concentrations, according to studies 1, 2. There are numerous conventional method adopted to minimize the pathogenic nature of phenylic acid (C6H5OH) in water resources available in India, and the permissible limit in drinking water is 1ppb (0.001 ppm). To oxidise various functional groups in synthetic organic chemistry, a variety of oxidants have arisen 3-5. Due to the two-nitrogen atoms present, the pyrazine moiety has been found to have biological activity.
When the Cr(VI) is employed as an oxidizing agent it is aggressive and possess serious threat to all living creatures. The highly reactive nature is reduced by linking it with a heterocyclic nuclei and therefore its toxic nature is also minimized. By this way the pyrazinium chlorochromate is prepared and the aggressive nature is passified. They have been proven to be incredibly mild, selective, regiospecific, and regioselective 9, 10, and they help in the oxidation of Cr+6 to Cr+3, which is non-toxic to the environment7, 8 and many scientists working in organic chemistry11, 12 employ pyrazinium chlorochromate (PzCC), one of its kind. The oxidation of phenol and substituted phenols has drawn the attention of numerous researchers13, 14. The literature review revealed that there are no known reports of pyrazinium chlorochromate in acetic acid oxidising phenols. The reactivity, correlation, and oxidation of a few meta and para substituted phenols were attempted to be studied utilising chromium (VI) oxidant in aqueous acetic acid medium.
Materials and Methods
The standard procedure was used to prepare the pyrazinium chlorochromate15, while the remaining chemicals were AnalaR grade, the phenols with meta and para replacements were obtained and distilled prior to use. The absorption of the phenylic acid was greater compared to pyrazinium chlorochromate (PzCC), so pseudo first order conditions were applied. The reaction was experimented at a fixed temperature of about 0.1K and the variation in the concentration of the oxidant [PzCC] was studied using colorimeter (470 nm). The products or reactants weren’t absorbed by this wavelength. Linear relationship with r = 0.991 was observed for 85% completion of the reaction from the log absorbance of the reactant with time. Less than 3% of the kinetic runs could be repeated. From the study for the oxidant (pyrazinium chlorochromate) first order kinetics was recorded and for all the substituted phenols, fractional order kinetics was seen. During the kinetic measurements for all the runs, one mole of pyrazinium chlorochromate consumed two moles of phenylic acid. Infrared, ultraviolet, and mass spectroscopy were used to verify the produced quinone after the solvent had been evaporated.
Results and Discussion
For the substituted phenols, rates and kinetic data were obtained. The oxidant’s reaction was first-order, and the plot’s linearity and high correlation coefficient (r = 0.991) were also favorable. With a slope value of 0.667, the log k1 against log [S] plot showed that the reaction rate increased as the substrate concentration increased. The addition of sodium perchlorate (NaClO4), which must affect the medium’s ionic strength but has no impact on the reaction’s pace, confirms the existence of ionic species in the slow phase. The reaction speed increased with the increase in the perchloric acid concentration [H+]. The presence of a protonated oxidant in the reaction was established with the regression value of 0.993 on log k1 vs log [acid] plot. The relationship between log k1 and 1/D was plotted (r = 0.999, B = +11.92) and showed that the rate increased as the percentage of acetic acid rose. The interface concerning the neutral molecule and the ion is thus shown to occur. Acrylonitrile, a radical scavenger, had no impact on the rate, excluding the idea of a free radical process. The reaction’s rate constant (k) slowly decreased with an increase in [MnSO4] due to the shift of two electrons. On the basis of these indications, the following process was hypothesized, and a corresponding rate rule was established.
Oxidation Process and the Mechanism
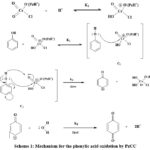 |
Scheme 1: Mechanism for the phenylic acid oxidation by PzCC |
Rate Equation
Influence of Various para and meta Substituents on the Speed of the Reaction
The reaction rate constant was determined for the substituted phenols (meta-Cl, meta -NO2, meta -Br, meta -CH3, para-CH3, para -COOH, para -NO2, para -Cl, para -OCH3 and para -Br) at four different temperatures viz., 20 0C, 30 0C, 40 0C and 50 0C and the thermodynamic factors and activation parameters were considered from the Eyring’s plot. The intermediate state of phenylic acid solvation was evidenced from the negative values of entropy of activation (ΔS#) difference as specified in the table 1. The ΔG# values was discovered to be small, specifying that pyrazinium chlorochromate oxidised all of the substituted phenols in a coordinated manner. The linear free energy relation (LFER) is applied to a set of reactions in which the change in activation entropy is proportional to the change in activation enthalpy. The isokinetic relationship or compensation law given by Hammett relates the enthalpies at various temperature by the following equation16,
ΔH# = ΔHo# + β ΔS#
The isokinetic plot between ΔH# versus ΔS# proved to be linear with r = 0.956 and the isokinetic temperature (β) was 335.14 K. Low Ea values and a shift in activation enthalpy (ΔH#) point to a coordinated process. We can deduce from the linear correlation that all the meta and para substituted phenylic acids follow the identical mechanism17. The decline in the entropy of activation (ΔS#) signifies the transition state is widely solvated owing to the amplified polarization. The following Exner relation criticized the linear correlation between ΔH# and ΔS#,
log (k1) T1 = a + b log (k1) T2
Where T2 > T1
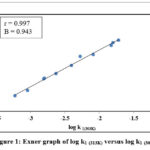 |
Figure 1: Exner graph of log k1 (313K) versus log k1 (303K) |
The plot of log k1(313K) versus log k1(303K) is undeviating with r = 0.997, specifies that all the substituents show a common mechanism (Figure 1).
Table 1: Thermodynamic and Activation Limits for the Oxidation of Phenols and substituted phenols by PzCC
|
S.No. |
Substituents |
Order w.r.to [S] |
k1 x 10-4 (s-1) |
ΔH# (kJ mol-1) |
(-) ΔS# (JK-1 mol-1) |
ΔG# at 303 K (kJ mol-1) |
Ea at 303 K (kJ mol-1) |
r |
|||
|
293 K |
303 K |
313 K |
323 K |
||||||||
|
1 |
-H |
0.667 |
3.73 |
5.93 |
8.17 |
10.81 |
10.92 ± 1.51 |
193.97 ± 2.8 |
69.69 ± 1.8 |
13.44 ± 1.51 |
0.995 |
|
2 |
p-Br |
0.447 |
7.71 |
8.97 |
11.12 |
13.92 |
5.66 ± 1.49 |
209.52 ± 3.1 |
69.14 ± 1.9 |
8.18 ± 1.49 |
0.991 |
|
3 |
p-Cl |
0.534 |
11.07 |
15.41 |
22.11 |
30.18 |
10.40 ± 1.53 |
191.99 ± 2.9 |
68.57 ± 2.1 |
12.92 ± 1.53 |
0.999 |
|
4 |
m-CH3 |
0.528 |
10.72 |
15.80 |
23.12 |
33.79 |
11.97 ± 1.48 |
186.75 ± 2.8 |
68.55 ± 2.0 |
14.49 ± 1.48 |
0.999 |
|
5 |
m-Cl |
0.597 |
18.12 |
23.37 |
30.39 |
40.69 |
8.06 ± 1.54 |
198.22 ± 3.2 |
68.12 ± 2.2 |
10.58 ± 1.54 |
0.998 |
|
6 |
p-CH3 |
0.624 |
31.87 |
39.21 |
49.11 |
61.67 |
6.41 ± 1.53 |
201.78 ± 3.1 |
67.55 ± 1.9 |
8.93 ± 1.53 |
0.998 |
|
7 |
p-COOH |
0.485 |
38.54 |
53.14 |
83.91 |
125.73 |
12.48 ± 1.46 |
180.50 ± 3.0 |
67.17 ± 1.8 |
14.99 ± 1.46 |
0.996 |
|
8 |
m-Br |
0.497 |
70.68 |
81.63 |
92.54 |
106.94 |
3.58 ± 1.48 |
208.57 ± 2.8 |
66.78 ± 2.2 |
6.04 ± 1.48 |
0.998 |
|
9 |
p-NO2 |
0.592 |
137.01 |
147.54 |
160.12 |
173.74 |
1.60 ± 1.49 |
212.93 ± 2.9 |
66.12 ± 2.1 |
4.12 ± 1.49 |
0.997 |
|
10 |
p-OCH3 |
0.569 |
134.43 |
157.32 |
187.74 |
229.18 |
4.95 ± 1.47 |
201.63 ± 3.1 |
66.04 ± 2.2 |
7.47 ± 1.47 |
0.994 |
|
11 |
m-NO2 |
0.380 |
170.51 |
189.49 |
220.12 |
267.79 |
3.54 ± 1.52 |
205.55 ± 3.2 |
65.82 ± 1.9 |
6.06 ± 1.52 |
0.991 |
[PzCC] = 0.5 x 10-3 mol dm-3 [Phenylic acid] = 1.5 x 10-2 mol dm-3 [H+] = 14.0 x 10-1 mol dm-3
CH3COOH : H2O = 60:40 (v/v)
Catalytic Effect
The catalytic effect was observed from the Hammett relation of phenols with the substituent constants (σ), showed non-linearity with a “V” shape curve. This implies that electron donating and electron removing substituents both speed up the reaction rate by producing negative and positive values, respectively (Table 2). This helps to confirm the involvement of electron deficient transition state in the common oxidation mechanism. The oxidation involves electron-withdrawing groups, negative charge is lost, or positive charge is produced on the intermediates.
Table 2: Catalytic Effect of Substituted Phenols
|
Substituent’s |
ρ |
k1 x 10-4 (s-1) |
R |
log k1 |
σ (substituent constant) |
|
para-OCH3 |
-ρ (B = -5.1174) (r = 0.9964) |
157.32 |
0.988 |
-1.8032 |
-0.27 |
|
para-CH3 |
39.21 |
0.994 |
-2.4066 |
-0.17 |
|
|
meta-CH3 |
15.80 |
0.990 |
-2.8013 |
-0.07 |
|
|
-H |
5.93 |
0.991 |
-3.2269 |
0.00 |
|
|
-H |
+ρ (B = +2.047) (r = 0.940) |
5.93 |
0.991 |
-3.2269 |
0.00 |
|
para-Br |
8.97 |
0.998 |
-3.0470 |
0.23 |
|
|
para-Cl |
15.41 |
0.990 |
-2.8122 |
0.23 |
|
|
meta-Cl |
23.37 |
0.999 |
-2.6313 |
0.37 |
|
|
para-COOH |
53.14 |
0.984 |
-2.2746 |
0.45 |
|
|
meta-Br |
81.63 |
0.998 |
-2.0882 |
0.39 |
|
|
para-NO2 |
147.54 |
0.993 |
-1.8311 |
0.78 |
|
|
meta-NO2 |
189.49 |
0.991 |
-1.7224 |
0.71 |
The Hammett Plot of Non-Linearity
The Hammett constant (σ) and reaction rate constant (k1) for the para– and meta-substituted phenols produced a “V” shaped, upward curve (figure 2) with a positive slope value. The variation in the slow step is due to the nature of the substituted group in phenol and nature of the intermediate state is the main reason for non-linearity in Hammett plot. The electron contributing groups was lined up on one side of the curve and the electron accommodating groups fall on the other side of the curve.
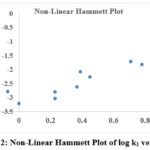 |
Figure 2: Non-Linear Hammett Plot of log k1 versus σ |
Evaluation of Antibacterial Activity
Pyrazinium chlorochromate’s antibacterial activity was tested using the well diffusion technique on Mueller-Hinton agar (MHA). The microorganisms were collected from Trichy Analytical Lab and Research Institute (Eumic). Some bacteria like Proteus Vulgaris, Streptococcus, Entrococcus, Micrococcus, Bacillus Cereus, Mycobacterium Tuberculosis, and fungilike Trichoderma, A.niger, Candida Albican and, Fusarium, were used as references for the antibacterial assay of the oxidant. Mueller-Hinton agar plates were inoculated with bacterial strain under sterile condition and wells of diameter (6 mm) were filled with 25 µl to 100 µl in DMSO of the test samples (PzCC) and incubated at 37°C for 25 to 28 hrs. The diameter of the inhibitory zone growth was assessed after the incubation time. Single colonies on agar plates were grown for 18 to 24 hours to produce a bacterial solution with a turbidity of 0.5 McFarland (equivalent to 1.5×108 colony-forming units (CFU)/ml)18, 19. The control was Ciprofloxacin, a common antibiotic. In the case of gram positive strains of Mycobacterium Tuberculosis (20 mm/ml), the compound PzCC showed good impedance, which was comparable to the control, and the inhibition of Proteus Vulgaris (25 mm/ml) was larger than that of Ciprofloxacin as specified in the figure 3.
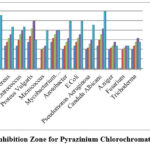 |
Figure 3: Inhibition Zone for Pyrazinium Chlorochromate (PzCC) |
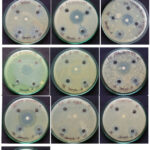 |
Figure 4: Agar well diffusion method – Inhibition zone for PzCC |
Conclusion
The investigation of oxidation reaction mechanism of phenol using pyrazinium chlorochromate proved the order followed first order kinetics for [O], fractional order for the [S] and [H+]. The rate of reaction did not vary as the medium’s ionic strength increased. Though the ionic strength contributed by the [NaClO4] of the reaction medium was raised, the reaction speed remained unchanged. With a drop in the medium’s dielectric constant (D), the reaction rate increased. When acrylonitrile was added, the free radical process was not detected. The presence of Mn (II) ions in the oxidation response was demonstrated by the addition of manganous sulphate. The activation and thermodynamic parameters ΔH#, ΔS#, ΔG# and Ea were evaluated for all the substituted phenols under investigation. The isokinetic plot (ΔH# versus ΔS#) showed linearity with r = 0.956 and the isokinetic temperature (β = 335.14 K) and non-linearity in the Hammett plot was evidenced from the reaction mechaism and the rate law. The antibacterial studies for the prepared compound exhibited higher resistance to few gram positive bacteria such as Entrococcus (20 mm/ml), Mycobacterium Tuberculosis (20 mm/ml) gram negative bacteria E.Coli (18 mm/ml) and for the fungi A.niger (14 mm/ml) and Trichoderma (16 mm/ml) respectively.
Acknowledgment
I wish to encompass my sincere thanks and gratitude to the management of Nehru Memorial College, my respected guide, Dr. K. Anbarasu, Assistant Professor in Chemistry, for his untiring help and encouragement.
Conflict of Interest
There is no conflict of interest.
References
- Rapti, I.; Bairamis, F.; Konstantinou, I. Photochem. 2021, 1, 358–370. https://doi.org/10.3390/photochem1030023
CrossRef - Antonopoulou, M.; Giannakas, A.; Konstantinou, I. Int. J. Photoenergy. 2012, 1, 358–370. https://doi.org/10.1155/2012/520123
CrossRef - Patil, S.G.; Joshi, S.B. Asian J. Chem. 2002, 14, 130-134.
- Ravishankar, M.; Sekar, K.G., Palaniappan. AN. Afinidad. 1998, 477, 357-362.
- Ahmed, F.; Omniah, S.; Moataz, M. J. Mol. Struct. 2021, 1229, 129495. doi.org/10.1016/j.molstruc.2020.129495
CrossRef - Jing C.; Zuo-Xiang Z,; Wei-Lan X.; Dan W.; Yu H. Ind. Eng. Chem. Res.2011, 50 (20), 11755-11762. DOI: 10.1021/ie2012714
CrossRef - Ziarani, G.M.; Aleali, F.; Lashgari, N. RSC Adv. 2016, 56, 50895-50922. https://doi.org/10.1039/C6RA09874F
CrossRef - . K. Ilavenil, A. Kasthuri and K Anbarasu, RASAYAN J. Chem. 2022, 15(3), 1660-1667. http://doi.org/10.31788/RJC.2022.1536920
CrossRef - Meenakshisundram, S.; Markkandan, R. Transit. Met. Chem. 2004, 29, 136 -143. https://doi.org/10.1023/B:TMCH.0000019410.94049.6b
CrossRef - Thirumoorthi, A.; Bhuvaneshwari, S.; Elango, K. P. Int. J. Chem. Kinet. 2007, 39(6), 362-369. https://doi.org/10.1002/kin.20255
CrossRef - Sekar, K.G.; Prabakaran, A. Oxid. Commun. 2008, 31(3), 613-622.
CrossRef - Sekar, K.G.; Anbarasu, K. Croat. Chem. Acta. 2009, 82, 819–823.
- Zhijiang, L.; Jay, G. Environ. Pollut. 2013, 180, 214-220. https://doi.org/10.1016/j.envpol.2013.05.047
CrossRef - Gupta, P.; Kothari, S. J. Chem. Sci. 2001, 113(2), 103-108. https://doi.org/10.1007/BF02704182
CrossRef - Davis, H.B.; Sheets, R. M. Heterocycles. 1983, 20, 2029.
CrossRef - Exner, O. Collection of Cell. Czech. Chem. Commun. 1964, 29(5), 1094-1113. https://doi.org/10.1135/cccc19641094
CrossRef - Saradamani, P.R.; Jegannathan, V. Indian J. Chem. 1990, 1(4), 700-702.
- Ze-Hua C.; Hui-Ling H.; Shuai-Bin W.; Chun-Liu D.; Si-Ya L.; Ti-Jiang S.; Liang-Xing F.; Xiao-Ping L.; Ya-Hong L.; Jian S. Antibiotics. 2021, 10 (4), 463. https://doi.org/10.3390/antibiotics10040463
CrossRef - Shoaib M.; Ganbarov K.; Ismiyev A.; Babayeva I.; Hussain I. Microbiology and Biotechnology Pages, 2021, 1(1), 1.

This work is licensed under a Creative Commons Attribution 4.0 International License.










