Synthesis, Characterization, Fluorescence, Electrochemical, Antimicrobial and Cytotoxic studies of Cobalt (II), Nickel (II) and Copper(II) complexes with N-4-Methylphenyl (2,4-dihydroxy acetophenylideneimine)
Amala Subbiah and Santhi Sambamoorthy
PG and Research Department of Chemistry, Seethalakshmi Ramaswami College Tiruchirappalli 620 002, Tamil Nadu, India.
Corresponding Author E-mail: santhi.muthuraman@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/3404039
Article Received on : 15-05-2018
Article Accepted on : 06-08-2018
Article Published : 27 Aug 2018
N-4-Methylphenyl (2,4-dihydroxy acetophenylideneimine) (NMEPDHAPI) complexes of Co(II), Ni(II) and Cu(II) have been synthesized and characterized. A study on their electrochemical, fluorescence and biological behaviour have also been performed. The complexes have been characterized on account of metal estimation studies, conductance behaviour, magnetic property, IR, UV-visible, EPR(Liquid nitrogen temperature) spectral studies and thermal analysis. The 1:2 metal : ligand complex formation is authenticated from the analytical data. Based on the various studies, the Co(II) complex has been recommended for octahedral geometry and a square planar geometry has been prompted for Cu(II) and Ni(II) complexes. The electro chemical property of the complexes have been studied by cyclic voltammetry. The Schiff base and its complexes have been scrutinized against bacterial and fungal pathogens. The complexes have been found to be more active than the Schiff base towards most of the pathogens studied. Invitro cytotoxicity of the copper complex of NMEPDHAPI has been screened against breast cancer cell lines (MCF-7). The IC50 value recommend that the copper complex has notable cytotoxicity against breast cancer cell line (MCF-7).
KEYWORDS:Antimicrobial; Cyclic Voltammetry; Fluorescence; Schiff base.
Download this article as:| Copy the following to cite this article: Subbiah A, Sambamoorthy S. Synthesis, Characterization, Fluorescence, Electrochemical, Antimicrobial and Cytotoxic studies of Cobalt (II), Nickel (II) and Copper(II) complexes with N-4-Methylphenyl (2,4-dihydroxy acetophenylideneimine). Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Subbiah A, Sambamoorthy S. Synthesis, Characterization, Fluorescence, Electrochemical, Antimicrobial and Cytotoxic studies of Cobalt (II), Nickel (II) and Copper(II) complexes with N-4-Methylphenyl (2,4-dihydroxy acetophenylideneimine). Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48765 |
Introduction
The metal complexes with azo linkage have been explored remarkably during the last few decades.1-3 Biological resemblance of Schiff bases is due to the structural resemblance of peptide bonds in protein.4 Such type of metal complexes exhibit many types of applications like catalysis,5 pharmaceuticals6 and molecular based materials.7 The continuous need for renewed anti-cancer drug has developed chemotherapeutic research depending upon the consume of metallic elements since this must have high potential activity and low side effects.8 A well known anticancer drug is cisplatin. Unfortunatelty cisplatin has number of side effects, which limit the clinical use.9 Therefore finding a new metal based drug with more activity and less side toxicity is a thurst area of research.10 The present report involves the synthesis of three transition metal complexes based on the Schiff base NMEPDHAPI. All three have been characterized from different spectral studies like IR, UV-visible and EPR in Liquid nitrogen temperature. The redox behavior from Cyclic voltammetry studies revealed the catalytic application of complexes and fluorescence property indicates the sensor applicability of the complexes. Both the ligand and the complexes have been screened against bacterial and fungal pathogens. Anticancer activity (Invitro cytotoxicity) of the copper complex of NMEPDHAPI has been inquired towards breast cancer cell lines (MCF-7).
Experimental
Materials and Methods
IR spectra were performed in KBr medium in FT-IR spectrophotometer (model : Shimadzu IR affinity). Measurements were recorded on powder samples at 298K. Thermal analysis was done by using the instrument (Perkin Elmer Diamond TG/DTA) from 400C to 7400C at the heating range about 200C/min. The EPR spectra were recorded using JEOL model JES FA200 ESR spectrometer in liquid nitrogen temperature. The UV-spectra of the Schiff base and its complexes were taken using Perkin Elmer Spectrometer provided with quarts cells in DMSO medium. The electrical conductance of the synthesized complexes were determined in 10-3 M solution of DMSO using a conductivity bridge of Elico make and a dip type conductivity cell. The percentage of metal was estimated by the standard methods. The magnetic susceptibility were ascertained in a Gouy Balance at room temperature. Electrochemical behaviour were measured at room temperature in an air tight three electrode cell by using glassy carbon electrode as a working electrode, a platinum wire served as the counter electrode and a Ag/AgCl in a saturated KCl solution as reference electrode. The electrochemical reactions were carried out using tetrabutylammonium perchlorate (0.11M) as a supporting electrolyte with a scan rate of 0.2V/s. The fluorescence spectra were analysed by using Jasco Spectro Fluorimeter (Model FP-8200) at room temperature by maintaining the concentration at 10-4M in DMSO.
Synthesis of Complexes
The Schiff base N-4-methylphenyl-(2,4-dihydroxyacetophenylideneimine) (NMEPDHAPI) has been synthesized, characterized through data from spectral studies. Its structure was established by single crystal XRD analysis by the authors in their previous article.11 The ligand NMEPDHAPI (0.2 mmol) and the metal salt (0.1mmol) (cobalt acetate, Nickel chloride & copper chloride) were dissolved in 100 ml of ethanol and allowed to react for 6 hours at 60ºC. The complex formed was filtered, washed with water and dried. The complexes are found to be freely soluble in DMF and DMSO.
[Co(NMEPDHAPI)2(H2O)2]: Yield: 90%, M.p.>255°C. m.w(g/mol):575.5; Metal percentage found (calcd.): 9.82(10.24); IR (Solid state, cm-1 ): ν(O-H) 3439; ν(̶ C=N ̶ ) 1655; ν(C-O) 1257; ν(M-O) 604; ν(M-N) 459; UV (In DMSO, λmax /nm): 924 (4T1g(F) →4T2g; 4T1g(F) →4A2g(F); 4T1g (F)→4A2g(P)) ; Conductivity in DMSO(ᴧM): 18 ohm-1cm2mol-1.
[Ni(NMEPDHAPI)2 ]: Yield:85% , M.p.>260°C m.w(g/mol): 539.2; Metal percentage found (calcd.): 10.23(10.88); IR (Solid state, cm-1 ): ν(O-H) 3362; ν(̶ C=N ̶ ) 1641; ν(C-O) 1226; ν(M-O) 626; ν(M-N) 534; UV (In DMSO,λmax /nm): 527 (1A1g→3A2g; 1A1g→1A2g; 1A1g→1B1g) ; Conductivity in DMSO(ᴧM): 14 ohm-1cm2mol-1.
[Cu(NMEPDHAPI)2 ]: Yield: 86%, M.p.>250°C m.w(g/mol): 544.1; Metal percentage found (calcd.): 11.08 (11.68); IR (Solid state, cm-1): ν(O-H) 3439; ν(̶ C=N ̶ ) 1607; ν(C-O) 1246; ν(M-O) 623; ν(M-N) 468; UV (In DMSO, λmax /nm): 537nm(2B1g→2Eg; 2B1g→2B2g and 2B1g→2A1g); Conductivity in DMSO(ᴧM): 22 ohm-1cm2mol-1.
Antimicrobial activities
The biopotency of all the synthesized metal complexes and the Schiff base ligand has been examined by disc diffusion technique employing nutrient agar as medium. The bacterial and fungal pathogens chosen for the study were staphylococcus aereus, klebsiella aerogenes, candida albicans and aspergillus niger.
Anticancer Activities
The human breast adeno carcinoma cell lines (MCF7) was collected from National Centre for Cell Science (NCCS), Pune. The MTT assay method was implemented to evaluate the cytotoxicity.12,13 The % cell inhibition was inclined by applying the formula
% Cell Inhibition = 100- Abs (sample)/Abs (control) x100. Nonlinear regression graph was computed between % Cell inhibition and Log concentration. The IC50 was ascertained by employing GraphPad Prism software.
Results and Discussion
Characterisation of Complexes
FT-IR Spectra
In complexes the band assignable to the azomethine linkage is shifted from 1605cm-1 in the Schiff base to 1607-1655cm-1 in their metal complexes. This proves the binding between the metal and azomethine linkage.14 The nitrogen coordination may be further authenticated by the presence of new band in the order of 467-561 in the complexes corresponding to M-N bond which is absent in the ligand.15,16 The another coordination site is phenolic oxygen which is present in the ortho position to the imine group. This is evidenced by the emergence of new band correlating to metal-oxygen bond around 600cm-1 in the complexes.17,18 The C-O stretching vibration of phenolic group is noticed at 1282cm-1 in the Schiff base NMEPDHAPI. This band is displaced to lower frequency in the spectra of complexes authenticating
coordination.19
Electronic spectra and magnetic susceptibility
The UV spectrum of the Co(II) complex exhibit the transition at 924nm assignable to 4T1g(F) → 4T2g, 4T1g(F) → 4A2g(F) and 4T1g (F) 4A2g(P) transitions20 and the magnetic moment of 4.65 BM support the octahedral arrangement around Co(II) ion.21 The band at 527nm in Ni(II) complex is designated to the transitions from 1A1g → 3A2g , 1A1g → 1A2g and 1A1g → 1B1g respectively22 correlating to the square planar geometry around Ni(II) and is diamagnetic.23 In the Cu(II) complex the band at 537nm is ascribed to 2B1g → 2Eg; 2B1g → 2B2g and 2B1g → 2A1g transitions24 of Cu(II) with square planar geometry. The magnetic moment of 1.54 BM substantiate the square planar geometry25 around Cu(II). The UV spectra of the complexes are given in supplementary data (Figure S1-S3).
![Figure S1: UV spectra of [Co(NMEPDHAPI)2(H2O)2]](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig1s-150x150.jpg) |
Figure S1: UV spectra of [Co(NMEPDHAPI)2(H2O)2] |
![Figure S2: UV spectra of [Ni(NMEPDHAPI)2]](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig2s-150x150.jpg) |
Figure S2: UV spectra of [Ni(NMEPDHAPI)2] |
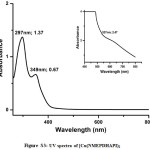 |
Figure S3: UV spectra of [Cu(NMEPDHAPI)2 |
EPR spectra
In liquid nitrogen temperature the complex [Cu(NMEPDHAPI)2] exhibits well defined peaks. The ground state of Cu(II) complex can be determined from the g value. In square planar complexes the unpaired electron lies in the dx2 – y2 orbital giving 2B1g as the ground state with g|| > g⊥. In our case for[Cu(NMEPDHAPI)2], g|| > g⊥ (2.30 > 2.03) which denotes the predominant existence of unpaired electron in dx2 – y2 orbital26 rather than dz2 . As a result the screening effect by the dz2 electrons is to a greater extent leading to elongation (Jahn teller distortion) finally detachment of two orbitals from the metal ion, resulting in square planor geometry. The epr spectra of the complex is shown in figure 1.
![Figure 1: EPR Spectra of [Cu(NMEPDHAPI)2]](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig1-150x150.jpg) |
Figure 1: EPR Spectra of [Cu(NMEPDHAPI)2]
|
Conductance Measurements
The molar conductance of the complexes were found to be ranging from 14 ohm-1 cm2 mol-1 to 22 ohm-1 cm2 mol-1. This evidences the non-electrolytic quality of the complexes.27 No change in conductance is observed when determined in different solutions. This further substantiate the neutral quality of all three complexes.
Metal estimation studies
The metal percentage of all the complexes were ascertained through standard methods.28 The amount of cobalt in complex was estimated by pyrolytic method. The quantity of nickel calculated by using titrimetric method. The copper percentage were found by colorimetric method. The values obtained are conforming with the values manipulated for the proposed structure of the complexes.
Thermal Analysis
In thermo gravimetric analysis of [Co(NMEPDHAPI)2(H2O)2], the first weight loss noticed in the temperature range 101-1420C (observed 5.97%, calculated 6.25%) is correlated with the displacement of two water molecules bound Co(II) ion. Another weight loss is in the temperature range of 156-5190C (observed 83.20%, calculated 83.85%) is suitable to the twofold decomposition of binary molecules of ligand NMEPDHAPI. In [Ni(NMEPDHAPI)] the weight loss ascertained in the temperature range 158-5420C (observed 89.67%, calculated 89.45%) is also due to the double step decomposition of two molecules of ligand. The thermogram of [Cu(NMEPDHAPI)] showed only one weight loss in the temperature range 136-3820C (observed 88.69%, calculated 85.09%) is owed to disintegration of couple of ligand moieties.29 The thermogram of all the complexes finally attains constant mass due to the dissipation of analogous metal oxides. The TGA/DTA curves are given under supplementary data (Figure S4-S6).
![Figure S4: TGA/DTA curve of [Co(NMEPDHAPI)2(H2O)2]](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig4s-150x150.jpg) |
Figure S4: TGA/DTA curve of [Co(NMEPDHAPI)2(H2O)2] |
![Figure S5: TGA/DTA curve of [Ni(NMEPDHAPI)2]](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig5s-150x150.jpg) |
Figure S5: TGA/DTA curve of [Ni(NMEPDHAPI)2] |
![Figure S6: TGA/DTA curve of [Ni(NMEPDHAPI)2]](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig6s-150x150.jpg) |
Figure S6: TGA/DTA curve of [Ni(NMEPDHAPI)2] |
From the above analytical data it is may be concluded that the cobalt complex is having octahedral geometry with two coordinated water molecules and nickel and copper complexes are in square planar geometry. The structure assigned for the complexes is depicted in figures 2 and 3.
![Figure 2: [Co(NMEPDHAPI)2(H2O)2]](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig2-150x150.jpg) |
Figure 2: [Co(NMEPDHAPI)2(H2O)2] |
![Figure 3: [M(NMEPDHAPI)2], M=Ni(II) and Cu(II)](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig3-150x150.jpg) |
Figure 3: [M(NMEPDHAPI)2], M=Ni(II) and Cu(II) |
Study on Redox Property
The cyclic voltammogram of [Co(NMEPDHAPI)2(H2O)2] exhibits two well defined quasi-reversible peaks. First reduction peak found in Epc = -0.680V with an associated oxidation peak placed at Epa = -0.338V and second reduction peak appearing in Epc = 0.132 V is associated with oxidation peak situated at Epa = 0.673 V. The value of ΔEp is 0.342V and 0.541V for first and second redox couples respectively and the ratio between oxidation and reduction peak current indicative of simple quasi-reversible one electron redox processes.30 The voltammogram of [Ni(NMPDHAPI)2] also shows one well denoted redox process. The reduction peak is viewed at EPc = -0.994V and the oxidation peak is noted at EPa = 0.730V. This couple is quasi-reversible and ratio between oxidation and reduction current suggests the process to be simple one-electron transfer.31 The voltammogram of [Cu(NMPDHAPI)2] displays one oxidation peak at EPa = -0.715V and the corresponding reduction peak at EPc = -1.224V . The another oxidation peak found in EPa = 0.603V and the respective reduction peak placed at EPc = 0.120V. The ΔEp values 0.509V and 0.483V shows that the redox processes are quasi-reversible. The correlation of oxidation to reduction peak height suggest that the the process is simple one electron process.32 The cyclic voltammograms are given in figure 4. The redox property of the complex may find the application in catalytic studies.
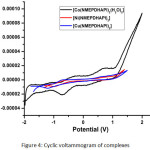 |
Figure 4: Cyclic voltammogram of complexes |
Fluorescence Property
The fluorescence spectra of all the synthesized compounds are shown in Figure 5. The emission peaks are noticed in the range of 357-359nm. Quenching of emission was observed in all the three complexes. This may be due to the predominance of PET process over CHEF effect.33 This quenching may lead to the chemo sensor utility of the Schiff base.
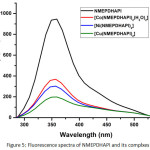 |
Figure 5: Fluorescence spectra of NMEPDHAPI and its complxes |
Antimicrobial activities
Antimicrobial activities of NMEPDHAPI and their complexes are given in Table 1. It is ascertained that the complexes are more active than the Schiff base towards almost all pathogens studied, which can be elucidated by Tweedy’s chelation theory.34 Lipophilic nature of the metal ion increases by chelation, which could facilitates to cross the lipid layer of the cell membrane. The coordination of metal ion to the Schiff base influence the magnetic property and conductance which may also be a cause for the extensive biological characteristics of the complexes. The azomethine bond also extend contribution for activity of the complexes.35 The maximum sensitiveness of the complexes is observed with the fungal pathogen candida albicans. The antimicrobial activity images are shown in Figures 6 and 7.
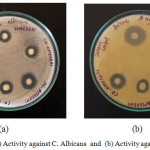 |
Figure 6: (a) Activity against C. Albicans and (b) Activity against A.Niger
|
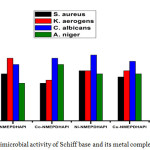 |
Figure 7: Antimicrobial activity of Schiff base and its metal complexes
|
![Figure 8: Invitrocytotoxicity of [Cu(NMEPDHAPI)] towards breast cancer cell line (MCF-7)](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Ama_fig8-150x150.jpg) |
Figure 8: Invitrocytotoxicity of [Cu(NMEPDHAPI)] towards breast cancer cell line (MCF-7)
|
Table 1: Antimicrobial activity of the ligand and its complexes.
| Compound /Complex | Zone of Inhibition in mm | |||
| Gram positive | Gram negative | Fungi | ||
| SA | KA | CA | AN | |
| NMEPDHAPI | 15 | 20 | 18 | 12 |
| [Co(NMEPDHAPI)2(H2O)2] | 12 | 13 | 20 | 18 |
| [Ni(NMEPDHAPI)2] | 16 | 16 | 21 | 15 |
| [Cu(NMEPDHAPI)2] | 14 | 16 | 19 | 15 |
| standard | 35 | 30 | 32 | 35 |
SA = S.aureus; KA = K.aerogenes; CA = C.albicans; AN = A.niger;. Solvent = DMSO;
Standard = Ciprofloxacin 5mg / disc for bacteria; Nystatin 100 units / disc for fungi
Anticancer Activities
The invitro cytotoxicity of copper complex were examined against breast cancer cell line (MCF-7) by MTT assay. The % of cell inhibition in different concentration shown in the figure 7 and table 2. The IC50 value of copper complex (27.61µM) compared with cis platin (4.5µM). From the IC50 value, it may be accomplished that the copper complex possess considerable cytotoxicity for breast cancer cell line (MCF-7).
Table 2: %Cell inhibition of [Cu(NMEPDHAPI)2] towards breast cancer cell line(MCF-7)
|
S. No |
Conc(µM) |
% Cell Inhibition |
|
1 |
0.25 |
1.053864 |
|
2 |
2.5 |
4.800937 |
|
3 |
25 |
37.00234 |
|
4 |
50 |
96.01874 |
|
5 |
100 |
100 |
Conclusion
The ligand NMEPDHAPI has been complexated with Cobalt(II), Nickel (II) and Copper (II). Analytical data exhibited that there is formation of 2:1 ligand to metal complexes. The low conductivity values intimated that the complexes are of neutral in nature. From various studies Co(II) complex has been suggested an octahedral geometry and a square planar geometry has been proposed for Ni(II) and Cu(II) complexes. The study of redox property and fluorescence property indicate that the complexes may find application as catalysts and chemosensors. The complexes are found to possess remarkable biopotency towards most of the pathogens studied which could be explained by chelation theory. The copper complex exhibits considerable cytotoxic behaviour towards breast cancer cell line (MCF-7).
Acknowledgements
The authors acknowledge their thanks to the Director, SAIF, IIT Madras, Chennai, SAIF, Cochin, St. Joseph’s College, Tiruchirappalli, SASTRA University, Thanjavur, Periyar College of Pharmaceutical Sciences, Tiruchirappalli and KMCH, Coimbatore for providing analytical support. The authors wish to express their thanks to the Secretary, Principal, Vice-Principal and faculty members of Chemistry, Seethalakshmi Ramaswami College, Tiruchirappalli, Tamil Nadu for providing laboratory facilities and support.
References
- Pattanayak, P.; Parua, S. P.; Patra, D.; Pratihar, J. L.; Brandão, P.; Felix, V.; Chattopadhyay, S. Synthesis, characterizations and structure of ortho metallated Pt(II) and Pt(IV) complexes: Oxidative addition to C,N,N,O coordinated Pt(II) complexes. Polyhedron, 2014, 70, 1-5.
CrossRef - Mitra, K.; Patil, S.; Kondaiah, P.; Chakravarty, A. R. 2-(Phenylazo)pyridineplatinum(II) Catecholates Showing Photocytotoxicity, Nuclear Uptake, and Glutathione-Triggered Ligand Release. Inorg. Chem., 2015, 54, 253-264.
CrossRef - Paul, N. D.; Samanta, S.; Mondal, T. K.; Goswami, S. Examples of reductive azo cleavage and oxidative azo bond formation on Re2(CO)10 template: isolation and characterization of Re(III) complexes of new azo-aromatic ligands. Inorg. Chem., 2011 50, 7886-7893.
CrossRef - Vishwakarma, P. K.; Mir, J. M.; Maurya R. M. Pyrone-based Cu(II) complexes, their characterization, DFT based conformational drift from square planar to square pyramidal geometry and biological activities. J. Chem. Sci., 2016, 128(4), 511- 522.
CrossRef - Decortes, A.; Castilla, A. M.; Kleij, A. W. Salen-complex-mediated formation of cyclic carbonates bycycloaddition of CO2 to epoxides. Angew. Chem. Int. Ed., 2010, 49, 9822- 9837.
CrossRef - Maurya, R. C.; Mir, J. M. Medicinal Industrial & Environmental Relevance of Metal Nitrosyl Complexes: A Review. Int. J. Sci. Eng. Res., 2014, 5, 305-321.
- Che, C. M.; Kwok, C. C.; Lai, S. W.; Rausch, A. F.; Finkenzeller, W. J.; Zhu, N.; Yersin, H. Photophysical properties and OLED applications of phosphorescent platinum(II) Schiff base complexes. Chem. Eur. J., 2010, 16, 233-247.
CrossRef - Garcia-Gimenez, J. L.; Gonzalez-Alvarez, M.; Liu- Gonzalez, M. J. Toward the development of metal-based synthetic nucleases: DNA binding and oxidative DNA cleavage of a mixed copper(II) complex with N-(9H-purin-6-yl)benzenesulfonamide and 1,10-phenantroline. Antitumor activity in human Caco-2 cells and Jurkat T lymphocytes. Evaluation of p53 and Bcl-2 proteins in the apoptotic mechanism. Inorg. Biochem., 2009, 103, 923-934.
CrossRef - Lopez, T.; Ortiz-Islas, E.; Guevara, P.; Gomez, E. Catalytic nanomedicine technology: copper complexes loaded on titania nanomaterials as cytotoxic agents of cancer cell. Int. J. Nanomed. 2013 8 581-592.
- Zuo, J.; Bi, C.; Fan, Y.; Buac, D.; Nardon, C.; Daniel, K. G.; Dou, Q. P. Cellular and computational studies of proteasome inhibition and apoptosis induction in human cancer cells by amino acid Schiff base-copper complexes. J. Inorg. Biochem., 2013, 118, 83-93.
CrossRef - Santhi, S.; Amala, S.; Highly Selective and Sensitive Dual Channel Schiff Base Chemosensors. 5th Annual International Conference on Chemistry, Athens, Greece, Abstract Book, 2017, 32, ISBN No. 978-960-598-161-7.
- Amala, S.; Santhi, S.; Suganya, R. Synthesis, Characterization, Fluorescence, Electrochemical and Biological Studies on Co(II), Ni(II) and Cu(II) complexes of N-4-methoxyphenyl (2,4-dihydroxyacetophenylideneimine).International Journal of Engineering and Applied Sciences, 2017, 4(10), 9-12.
- Mohamed, G. G.; Ibrahim, N. A.; Attia, H. A. E. Spectroscopic studies on some azo compounds and their cobalt, copper and nickel complexes. Spectrochim. Acta A, 2009,72, 610-615.
CrossRef - Sreedaran, S.; Shanmuga Bharathi; Kalilur Rahiman, A., Rajesh, K.; Nirmala, G.; Narayanan, V. Synthesis, spectral, magnetic, electrochemical and catalytic studies of cyclam-based copper(II) and nickel(II) complexes – effect of N-substitution. Journal of Coordination Chemistry, 2008, 61(22), 3594- 3609.
CrossRef - Al-Shaalan N. H. Synthesis, characterization and biological activities of Cu(II), Co(II), Mn(II), Fe(II), and UO2(VI) complexes with a new Schiff Base hydrazone: o-hydroxyacetophenone-7-chloro-4-quinoline hydrazone. Molecules, 2011, 16, 8629-8645.
CrossRef - Bassett, J.; Denney R. C.; Jeffery G. H.; Mendham. J.;, Vogel’s Textbook of Quantitative Inorganic Analysis including Elementary Instrumental Analysis, Longman Group Limited 1978, 322,461,740.
- Hammam, A. M.; EL-Gahami, M. A.; Khafagi, Z. A.; Al-Salimi, M. S.; Ibrahim, S. A. Synthesis and Characterization of Some New Antimicrobial Transition Metal Complexes with 1, 2, 4-Traizole-3-thione Schiff Bases. J. Mater. Environ. Sci., 2015, 6(6), 1596-1605.
- Shiekhl, R. A.; Rahman, I. A.; Malik, M. A.; Masudi S. M.; Luddin N. Synthesis, Spectral, Electrochemical and Biological Studies of Nitrogen Donor Macrocyclic Ligand and its Transition Metal Complexes.Int. J. Electrochem. Sci., 2012, 7, 12829-12845.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 1983, 65, 55-63.
CrossRef - Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; Gray-Goodrich, M.; Campbell, H.; Mayo, J.; Boyd. Feasibility of high flux anticancer drug screen using a diverse panel of cultured human tumour cell lines. Journal of the National Cancer Institute, 1991, 83, 757-766.
CrossRef - Halli, M. B.; Patil V. B. Synthesis, Characterization and DNA cleavage studies of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) complexes with benzofuran-2-carbohrdrazide Schiff bases. Indian Journal of Chemistry, 2011, 50A, 664-669.
- Vidya, V. G.; Sadasivan, V.; Meena, S. S.; Bhatt P. Synthesis, Spectral and Biological Studies of Complexes with Bidentate Azodye Ligand Derived from Resorcinol and 1-amino-2-naphthol-4-sulphonic Acid, Oriental Journal of Chemistry, 2018, 34(1), 45-54.
- Khalil, M. M. H.; Ismail, E. H.; Mohamed, G. G.; Zayed, E. M.; Badr, A. Synthesis and characterization of a novel schiff base metal complexes and their application in determination of iron in different types of natural water. Open Journal of Inorganic Chemistry, 2012, 2, 13-21.
CrossRef - Bhagat, T. M.; Swamy, D. K.; Deshpande, M. N. Synthesis and characterization of transition metal complexes with newly synthesized substituted benzothiazlole. J .Chem. Pharm. Res.,2012, 4, 100-104.
- Aliyu, H. N.; Ado, I. Studies of Mn (II) and Ni (II) complexes withSchiff base derived from 2-amino benzoic acid and salicylaldehyde. Biokemistri, 2011, 23(1), 9-16.
- Nejo A. A.; Kolawole G. A.; and Nejo A. O. Synthesis, characterization, antibacterial, and thermal studies of unsymmetrical Schiff-base complexes of cobalt(II). Journal of Coordination Chemistry, 2010, 63(24), 4398-4410.
CrossRef - Hasan M. R.;, Hossain M. A.; Salam M. A.; Uddin M. N. Nickel complexes of Schiff bases derived from mono/diketone with anthranilic acid: Synthesis, characterization and microbial evaluation . Journal of Taibah University for Science, 2016, 10, 766-773.
CrossRef - Santhi, S.; Sandhiya, S.; Ramya, R.; Amala, S. Synthesis Characterization and Biological Studies on N,N’-Ethylenebis-(2,4 Dihydroxy acetophenylideneimine) and its Complexes with Mn(II), Co(II), Ni(II) and Cu(II). The international journal of science and technoledge, 2014, 2(10), 102-106.
- Dhayabaran, V. V.; Prakash, T. D.; Renganathan, R.; Friehs, E.; Bahnemann, D. W. Novel Bioactive Co(II), Cu(II), Ni(II) and Zn(II) Complexes with Schiff Base Ligand Derived from Histidine and 1,3-Indandione: Synthesis, Structural Elucidation, Biological Investigation and Docking Analysis. Journal of fluorescence, 2017, 27(1), 135-150.
CrossRef - Selvameena, R.; Santhi, S.; Anusha, D.; Amala, S. Synthesis, Characterization and Biological Studies of N,N’-Bis(2-Hydroxynaphthalidene)-4-Ethylphenyl Methanediamine and its Complexes with Co(II),Ni(II) and Cu(II) The international journal of science and technoledge, 2014, 2(10), 107-112.
- Abdou, S. N.; Faheim, A. A.; Alaghaz A. N. M. A. Synthesis, Spectral Characterization, Cyclic Voltammetry, Molecular Modeling and Catalytic Activity of Sulfa-Drug Divalent Metal Complexes.Current Synthetic and Systems Biology, 2013, 2(2), 1000112.
- Kulkarni, A. D.; , Patil, S. A.; Badami P. S. Electrochemical Properties of some Transition MetalComplexes: Synthesis, Characterization and In-vitro antimicrobial studies of Co(II), Ni(II), Cu(II), Mn(II) and Fe(III) Complexes. Int. J. Electrochem. Sci., 2009, 4, 717-729.
- Li, Z. A.; Lou, X. D.; Yu, H. B.; Gin, J. G.; Imidazole-functionalized polyfluorene derivative as sensitive fluorescent probe for metal ions and cyanide. Macromolecules, 2008, 41, 7433-7439.
CrossRef - C. Anitha, C. C. D. Sheela, C. D.; Tharmaraj, P.; Shanmugakala, R. Studies on Synthesis and Spectral Characterization of Some Transition Metal Complexes of Azo-AzomethineDerivative of Diaminomaleonitrile, International Journal of Inorganic Chemistry, 2013, Article ID 436275, 1-10.
- Sonmez, M.; Metin, C.; Berber, I. Synthesis, spectroscopic and biological studies on the new symmetric Schiff base derived from 2,6-diformyl-4-methylphenol with N-aminopyrimidine. Eur. J. Med. Chem.,2010, 45, 1935-1940.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









