Using Biebersteinia Multifida Aqueous Extract, the Photocatalytic Activity of Synthesized Silver Nanoparticles
Abdolhossien Miri1, Seyedeh Raziyeh Mousavi2, Mina Sarani3 and Zohre Mahmoodi4
1Department of Pharmacognosy, Faculty of Pharmacy, Zabol University of Medical Sciences, Zabol, Iran.
2Department of Horticulture and Landscape, Faculty of Agriculture, University of Zabol, Zabol, Iran.
3Zabol Medicinal Plants Research Center, Zabol University of Medical Sciences, P.O. Box, 3333-669699, Zabol, Iran.
4Department of Cardiovascular Surgery, Zabol University of medical Science, Zabol, Iran.
Corresponding Author E-mail: minasarani64@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340342
Article Received on : August 31, 2017
Article Accepted on : February 22, 2018
Article Published : 03 May 2018
Nowadays synthesis of nanoparticles using plants and herbal products is significant by the many researchers. In this study, we report the synthesis of silver nanoparticles (Ag NPs) using aqueous extract of Biebersteinia Multifida at room temperature and its photocatalytic activity for degradation of methyl blue (MB) dye under visible light. The synthesized nanoparticles were characterized by using UV-vis spectroscopy, powder X-ray diffraction (PXRD) and transmission electron microscopy (TEM). Synthesized nanoparticles are spherical with 57 nm of particle size. Results photocatalytic activity of synthesized Ag NPs showed that degradation efficiency arrived to 92% in first 30 min.
KEYWORDS:Ag NPs; B. Multifida; Green Synthesis; Methyl Blue; Photocatalytic
Download this article as:| Copy the following to cite this article: Miri A, Mousavi S. R, Sarani M, Mahmoodi Z. Using Biebersteinia Multifida Aqueous Extract, the Photocatalytic Activity of Synthesized Silver Nanoparticles. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Miri A, Mousavi S. R, Sarani M, Mahmoodi Z. Using Biebersteinia Multifida Aqueous Extract, the Photocatalytic Activity of Synthesized Silver Nanoparticles. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45340 |
Introduction
Silver nanoparticles (Ag NPs) are considered vital in medicine and industry; therefore they are utilized for purposes such as antiseptics, covering medical instruments [1-5], biosensors [6], gene and drug carriers [7, 8], catalysis and electrical conductance [9]. The biologic and catalysis activity of Ag NPs depended on its specific surface area [9]. Due to the widespread use of Ag NPs, many different methods for their synthesis have been reported including gamma rays [10], ultraviolet rays [11], electrochemical reduction [12], and reducing agents such as sodium boron hydride (NaBH4) [13, 14], which are mostly expensive, toxic, time-consuming, and require special equipments. Since it can be concluded that a low cost, quick, and environmentally friendly method is needed for the synthesis of Ag NPs, using plants for this purpose would be a desirable suggestion.
Biebersteinia multifida DC (Geraniaceaae family) is a native plant of Iran, also called Chele Daq and Adamak, but grows in Syria, Armenia, Afghanistan, Lebanon and Central Asia as well [15]. Its active ingredients are Alkaloids, polysaccharides, polypeptides and Flavonoids such as Apigenin, Luteolin, and Tricetin that have antioxidant and antimicrobial effects [16]. Recent studies on B. multifida essential oil have indicated on the existence of (E)-nerolidol [17]. Due to the existence of the mentioned compounds, it is likely that B. multifida is capable of reducing metal ions and thus, it was utilized for the synthesis of Ag NPs.
Dyes use widely at industries and daily life in textile, paper and plastics and they enter to the environment and pollution it. So, dyes pollution is one of the challenges for researchers to eliminate them. The common methods used to remove industrial dyes are electro-coagulation, carbon sorption, redox treatment, flocculation, UV-photo degradation [18]. Reports have shown that metallic nanoparticles can act as photocatalytic agents [19-21]. Therefore, in the study we were investigated photocatalytic activity of synthesized Ag NPs using B. multifida extract on MB dye.
Materials and methods
Preparation of B. multifida extract
The root bark of B. multifida was collected from Borujen, Chaharmahal and Bakhtiari, Iran, in the spring of 2015. It was dried in shadow. The herbarium code 42094 was allocated to it by Herbarium of the Ferdowsi University of Mashhad (FUHM). The sample was extracted by using Maceration method. 10 gr of the sample was weighed and 100 ml of distilled water was added to it. Then it was placed on a rotating shaker at 100 rpm for 6 h. The crude extract was filtered by filter Whatman No.1 and the result (yellow solution) was kept in refrigerator with a temperature of 4-7ºC for the following steps.
Preparation of Ag NPs
Silver nitrate solution was added to the aqueous extract of B. multifida and the solution was shaken at 100 rpm for the defined time and temperature. The formation of a dark brown solution indicated that Ag NPs are produced. In this regard, the four parameters including volume of extract, salt concentration, temperature, and time on the formation of nanoparticles were analyzed. Thorough out all the tests, only one parameter was variable and the rest were fixed.
Photocatalytic activity of Ag NPs
In order to survey photocatalytic activity of Ag NPs, 25 mg of synthesized Ag NPs was added to 25 ml MB solution (15 mg/l). The mixture was stirred constantly for 30 min in darkness to ensure constant equilibrium of Ag NPs in the organic solution. Then, the suspension was put under the visible light. In the periods of 30 min, 3 ml of suspension was centrifuged at 4000 rpm for 5 min and absorbance spectrum of the solution was measured using a UV–visible spectrophotometer. The percentage degradation of MB was analyzed using below equation:
Formula 1
C0 is initial concentration of dye solution and Ct is the concentration of dye solution after exposure to the visible light.
Characterization of Ag NPs
Electronic spectra in the visible and ultraviolet regions were recorded in water as solvent through the use of UV-vis Spectrophotometer (Rayleighuv-2100 model, made in China). Powder X-ray diffraction (PXRD) of synthesized nanoparticles was recorded by utilizing the XRD device (X’Pert PRO MPD PANalytical Company model, made in Netherland). The image of nanoparticles was presented through the use of transmission electron microscopy (TEM) (Zeiss EM900 model, made in Germany).
Results and Discussion
The basis of synthesizing nanoparticles would be ion reduction and neutralizing the electric charge of particles. The changing color of solution from yellowish orange to brown indicates the formations of colloidal suspension of Ag NPs, which is due to silver ion reduction.
UV-vis spectroscopy
UV-vis spectroscopy is a vital method to determine the formation and stability of metal nanoparticles in an aqueous solution [22]. The Ag NPs show their highest peak of UV-vis absorption in the range of 400-500 nm [23]. The position and shape of the plasmon absorption depends on the size and shape of the particles and dielectric constant of its surrounding area [24]. A peak in the region of 427 nm was observed in the UV-vis spectrum of synthesized Ag NPs, which indicates on the formation of Ag NPs Figure 1.
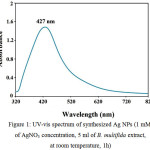 |
Figure 1: UV-vis spectrum of synthesized Ag NPs (1 mM of AgNO3 concentration, 5 ml of B. multifida extract, at room temperature, 1h) |
The effects of AgNO3 concentrations (0.5, 1, and 3 mM) on the synthesis of Ag NPs in 5 mL of aqueous extract of the plant were analyzed for 1 hour at room temperature. The maximum absorption bands of synthesized Ag NPs via silver nitrate concentrations of 0.5, 1, and 3 mM appeared in ranges of 464, 435 and 440 nm, respectively Figure 2. The survey of spectra have shown that with increasing the concentration of silver nitrate salt, the conversions of Ag+ to Ag0 increase as well and thus, the peak intensity increase and the solution color become heighten. Increasing Agº concentration can cause increasing in particle diameter, which would result in increasing absorption [25].
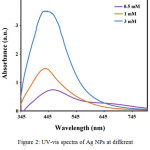 |
Figure 2: UV-vis spectra of Ag NPs at different concentrations of AgNO3
|
Through analyzing the effects of aqueous extract volume on Ag NPs synthesis, it was observed that by increasing the volume of extract, peak intensity increased as well and the spectrum got a blue shift Figure 3. The plant extract contains organic materials that act as reducing agents and particles stabilizer. When the extract volume increases, nanoparticles get surrounded with higher amounts of organic materials and the particles become heavier, so they settle and lose their nano form; therefore, it was perceived that the absorption intensity decreased by increasing the extract volume to 20 ml. Considering the observations, the most suitable extraction volumes would be 3 and 5 ml [26, 27].
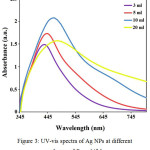 |
Figure 3: UV-vis spectra of Ag NPs at different volumes of B. multifida
|
The effects of reaction temperature (20, 40 and 60ºC) on the formation of Ag NPs (concentration of 1 mM and 1 h) suggest that by increasing the temperature, peak intensity increases as well and shifts toward lower wavelengths; therefore, there were probably more nanoparticles synthesis or the diameter of synthetic nanoparticles were increased Figure 4. [26].
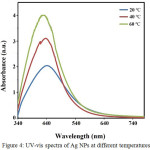 |
Figure 4: UV-vis spectra of Ag NPs at different temperatures |
PXRD Analysis
The powder X-ray diffraction (PXRD) of Ag NPs at a silver nitrate concentration of 1 mM and 5 mL of aqueous extract, for the duration of 1 hour at room temperature, is presented in Figure 5. This graph demonstrates 5 types of peaks at values of 041/38º, 3/44º, 5/64º and 66/77º, 79/85º in accordance with surfaces of (111), (200), (220), and (311) respectively [28, 29]. The peak observed in PXRD graph confirms the crystalline structure of nanoparticles. Moreover, the additional peaks that have appeared in this spectrum are silver chloride [28]. This figure illustrates a centered cubic structure for synthesized Ag NPs.
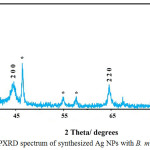 |
Figure 5: PXRD spectrum of synthesized Ag NPs with B. multifida extract
|
TEM Image
The TEM image of synthesized Ag NPs illustrate that they are equal in size according to nanoscale and uniformed in shape. Also, the image demonstrates that in optimal conditions, the particles size of synthesized Ag NPs is 57 nm Figure 6. The TEM image shows that synthesized nanoparticles have spherical shapes.
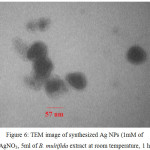 |
Figure 6: TEM image of synthesized Ag NPs (1mM of AgNO3, 5ml of B. multifida extract at room temperature, 1 h)
|
Photodegradation activity of MB
Photocatalytic activity of the prepared Ag NPs was surveyed using MB degradation model. MB is an aromatic dye that dissolves in water and has UV-vis absorption peak at 665 nm. The intensity of MB absorption is related to the concentration of MB. After reaction the reduction of MB causes decreasing of the absorption intensity. The result showed Figure 7 rapid decrease in MB color from 0 to 30 min, which become slower after 30 min. so that degradation efficiency arrived to 92% in first 30 min. Elemike et al was suggested the mentioned behavior is attributed to the porosity of Ag NPs [30]. Chen et al was reported that Ag NPs obtained using M. tinctoria degrade 29.5% MB in 24 h [31] and also, Elemike et al was reported it 68.7% [30]. In compared with mentioned report Ag NPs synthetized using B. multifida is more effective, so it has high porosity and can act as good electron donor.
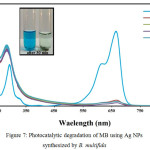 |
Figure 7: Photocatalytic degradation of MB using Ag NPs synthesized by B. multifida |
Conclusion
Silver nanoparticles are widely used in industrial and medical applications. The antibacterial properties of these particles have caused a revolution in medical science. They are utilized in healing wounds and injuries, as well as in the preparation of dressings, creams and covering medical devices such as catheters, artificial teeth, and surgical masks. Therefore, considering the needs of nanoparticles in medicine and industry, proposing a cheap, easy, quick, accessible, and non-polluting method to synthesize nanoparticles is a necessity. “Green” synthesis is a desirable method to prepare nanoparticles since it meets all of the mentioned demands. The results of this study showed that the aqueous extract of B. multifida root bark, as a reducing agent of silver ion, can synthesis nanoparticles in a short time with sizes less than 57 nm; while further studies displayed the good photocatalytic properties of the synthesized nanoparticles.
References
- Gauger, A.; Mempel, M.; Schekatz, A.; Schäfer, T.; Ring, J.; Abeck, D. Dermatology. 2003, 207, 15-21.
CrossRef - Rupp, E.M.; Fitzgerald, T.; Marion, N.; Helget, V.; Puumala, S.; Anderson, J.R.; Fey, P.D. Am J Infect Control. 2004, 32, 445–450.
CrossRef - Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; de Camargo, E.R.; Barbosa, D.B. Int J Antimicrob Agents. 2009, 34, 103–110.
CrossRef - Khatami, M.; Mehnipor, R.; Sobhani Poor M.H.; Jouzani, G.S. J Clust Sci. 2016, 27(5), 1601-1612.
CrossRef - Khatami, M.; Pourseyedi, S. IET Nanobiotechnol. 2015, 1–7.
- Xia, N.; Chen, Z.; Liu, Y.; Ren, H.; Liu, L. Sens Actuators B Chem. 2017, 243, 784–791.
CrossRef - Kasyanenko, N.; Bakulev, V.; Perevyazko, I.; Nekrasova, T.; Nazarova, O.; Slita, A.; Zolotova, Y.; Panarin, E. J Biotechnol. 2016, 236, 78–87.
CrossRef - Kumar, C.G.; Poornachandra, Y. Colloids Surf B Biointerfaces. 2015, 125, 110–119.
CrossRef - Tahir, K.; Nazir, S.; Li, B.; Khan, A.U.; Haq Khan, Z.U.; Ahmad, A.; Khan, Q.U.; Zhao, Y. J Photochem Photobiol B. 2015, 153, 261–266.
CrossRef - Ali, I.; Akl, M.R.; Meligi, G.A.; Saleh, T.A. Results in Physics. 2017, 7, 1319–1328.
CrossRef - Lei, Y.; Gao, G.; Liu, W.; Liu, T.; Yin, Y. Appl Surf Sci. 2014, 317, 49–55.
CrossRef - Thuc, D.T.; Huy, T.Q.; Hoang, L.H.; Tien, B.C.; Chung, P.V.; Thuy, N.T.; Le, A.T. Mater Lett. 2016, 181, 173-177.
CrossRef - Manivel, P.; Balamurugan, A.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, C. Spectrochim Acta A Mol Biomol Spectrosc. 2012, 95, 305–309.
CrossRef - Harish, B.S.; Uppuluri, K.B.; Anbazhagan, V. Carbohydr Polym. 2015, 132, 104–110.
CrossRef - Golshan, A.R.; Hassanzadeh, S.; Mojdekanloo, M.; Tayarani-Najaran, Z. Avicenna J Phytomed. 2016, 6, 671-677.
- Greenham, J.; Vassiliades, D.D.; Harborne, J.B.; Williams, C.A.; Eagles, J.; Grayer, R.J.; Veitch, N.C. Phytochemistry. 2001, 56, 87-91.
CrossRef - Monsef-Esfahani, H.R.; Amini, M.; Goodarzi, N.; Saiedmohammadi, F.; Hajiaghaee, R.; Faramarzi, M.A.; Tofighi, Z.; Ghahremani, M.H. DARU. 2013, 21.
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Spectrochim Acta A Mol Biomol Spectrosc. 2015, 146, 286–291.
CrossRef - Suvith, V.S.; Philip, D. Spectrochim Acta A Mol Biomol Spectrosc. 2014, 118, 526–532.
CrossRef - Saoud, K.; Alsoubaihi, R.; Bensalah, N.; Bora, T.; Bertino, M.; Dutta, J. Mater Res Bull. 2015, 63, 134–140.
CrossRef - Hosseini, S.J.; Aghaie, H.; Ghaedi, M. Orient J Chem. 2014, 30(4), 1883-1895.
- Naraginti, S.; Sivakumar, A. Spectrochim Acta A Mol Biomol Spectrosc. 2014, 128, 357–362.
CrossRef - Dyrba, M.; Miclea, P.T.; Schweizer, S. Radiat Meas. 2010, 45, 314–316.
CrossRef - Guzman, M.; Dille, J.; Godet, S. Nanomedicine. 2012, 8, 37–45.
CrossRef - Xu, W.; Jin, W.; Lin, L.; Zhang, C.; Li, Z.; Li, Y.; Song, R.; Li, B. Carbohydr Polym. 2014, 101, 961– 967.
CrossRef - Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Arabian J Chem. 2014, 7, 1131–1139.
CrossRef - Ahmed, S.; Saifullah, Ahmad, M.; Swami, B.L.; Ikram, S. J Radiat Res Appl Sci. 2016, 9, 1-7.
- Miri, A.; Sarani, M.; Rezazade Bazaz, M.; Darroudi, M. Spectrochim Acta A Mol Biomol Spectrosc. 2015, 141, 287–291.
CrossRef - Miri, A.; Dorani, N.; Darroudi, M.; Sarani, M. Cell Mol Biol. 2016, 62, 46-50.
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Ehiri, R.C.; Nnaji, N.J. Mater Sci Eng C. 2017, 75, 980–989.
CrossRef - Chen, Y.; Li, N.; Zhang, Y.; Zhang, L. J Colloid Interface Sci. 2014, 422, 9–15.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









