Adsorption of Lead (Ii) Ions by Ecofriendly Copper Oxide Nanoparticles
Sreekala G *, Fathima Beevi A and Beena B
Nanoscience Research Lab, Department of Chemistry K.S.M.D.B.College, Sasthamcotta-690521, Kollam, Kerala, India
Corresponding Author E-mail: sreekalamohankumar@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350615
Article Received on : 14-Oct-2019
Article Accepted on : 15-Nov-2019
Article Published : 21 Nov 2019
The present investigation is on the application of green synthesized CuO nanoparticles for elimination of lead (II) from waste water. Nano CuO was prepared from aqueous copper acetate solution and aqueous leaf extract of Simarouba glauca plant. The prepared nano CuO was characterized by XRD, FT-IR, UV, SEM and TEM. The nano CuO synthesized by this method was spherical in shape with particle size nearly 20 nm. The adsorption of lead (II) ions on nano CuO under various parameters such as amount of catalyst, concentration of metal ion and pH were studied using batch adsorption experiments. Experimental results indicate that the green synthesized CuO nanoparticle is a very good adsorbent for the efficient removal of Lead (II) from waste water. Optimum conditions for 95% adsorption of Pb2+ on CuO nanoparticle are pH 6, amount of catalyst 0.05g and concentration of metal ion 10mgL-1.
KEYWORDS:Green synthesis; Copper oxide nanoparticles; Simarouba glauca; Adsorbent; Lead (II) ion
Download this article as:| Copy the following to cite this article: Sreekala G, Beevi A. F, Beena B. Adsorption of Lead (Ii) Ions by Ecofriendly Copper Oxide Nanoparticles. Orient J Chem 2019;35(6). |
| Copy the following to cite this URL: Sreekala G, Beevi A. F, Beena B. Adsorption of Lead (Ii) Ions by Ecofriendly Copper Oxide Nanoparticles. Orient J Chem 2019;35(6). Available from: https://bit.ly/2D5I9BS |
Introduction
Now a day’s an increase in industrial globalization produces a number of pollutant into the environment. Heavy metals are the major inorganic water pollutants in the world due to the high toxicity on human[1].Heavy metal ions are dispersed into the environment from different processes like metallurgy, mining, battery making industries etc. The heavy metal in waste water and surface water is becoming a major health hazard. Pb (II) is one of the highly toxic materials which have adverse effects on human being. Exposure of very low levels of lead to children can cause different types disabilities like learning disabilities, reduced IQ, behavioral problems, etc. But at high levels, a child may become mentally retarded, fall into a comma stage and even die from lead poisoning. Exposure of lead in adults cause increase in blood pressure, muscle and joint pain, and several types of disorders [2]. Because of these health problems it is very important to eliminate such a dangerous heavy metal ion in waste water before discharging it into the environment. The permissible limit of Pb (II) on drinking water is 0.015mg/l as per EPA [3].Hence it is very urge to eliminate such a metal ion from wastewater before it can be discharged. The different methods have been employed for the removal of metal ions from aqueous solutions are precipitation, evaporation, electro deposition, ion exchange, membrane separation, coagulation etc[4]. The disadvantages of these methods are secondary pollution, high cost, high energy input, large quantities of chemical reagents, poor treatment efficiency at low metal ion concentration especially when the concentration of metals in wastewater is low (<100 ppm)[5,6]. Hence it is very urge to develop an efficient and low-cost method of elimination of metal.
Adsorption is an efficient conventional technique to remove heavy metals and organic pollutants from aqueous solutions. A number of adsorbents for waste water treatment have been commercialized or are being developed [7]. Most of these adsorbents are highly porous and have sufficient surface area for adsorption. Thus it is very important to develop an adsorbent with large surface area and small diffusion resistance to meet the practical and environmental applications [8].
Copper oxide nanoparticles have wide range of applications in various fields like electronics, sensors [9], antioxidants, heterogeneous catalysts [10], and in the field of biomedicine [11]. In the common chemical method of preparing nanoparticles find opposite effects in medicinal application due to the adsorption of hazardous chemical on the surface of nanoparticles. Hence researchers are constantly tried to make synthesizing nanoparticles by green approach to reduce the toxicity. Very recently researchers used green method of synthesizing various nanoparticles by plants such as neem [12], alfalfa [13, 14], Cinnamomum camphora [15], Emblica officinalis [16], lemon grass [17], tamarind [18] and Saraca indica [19]. Copper oxide nanoparticles have very interesting biological and mechanical properties [20]. These versatile properties and applications developed by green method make copper oxide nanoparticle a very important material in waste water treatment.
Simarouba glauca is an evergreen tree and has a long history of herbal medicine [21] metabolites which include alkaloids, steroids, flavonoids, terpenoids, glycoside, saponia, tannins, phenolic compounds and so on [22]. These metabolites can act as reducing agent as well as capping agent. Simarouba glauca was reported with medicinal applications such as haemostatic, antihelmenthic, antiparasitic, antidysentric, antipyretic and anticancerous [23]
Reports on biosynthesis of CuO nanoparticles are very few. Green method for the synthesis of CuO nanoparticles is simple, low cost, not time consuming, single step and eco-friendly. Therefore, this present work was planned to investigate the adsorption of lead (II) ions in solutions with nano copper oxide catalyst prepared from aqueous leaves extract of Simarouba Glauca plant.
Materials and methods
Materials
The only chemical used in this present study was analytical grade Copper (II) acetate monohydrate [(CH3COO) 2Cu.H2O].
Methods of Synthesis of CuO nanoparticles
Preparation of Leaf Extract
Simarouba Glauca leaves were collected, washed, dried in shade and pulverized. 20 g of the pulverized leaves were treated with 300 ml deionised water and heated in a microwave oven at 100W for 30 minutes. Thereafter the solution was filtered using Whatman 1 and filtrate obtained was collected. This freshly prepared leaf extract was used for synthesizing copper oxide nanoparticles.
Preparation of CuO nanoparticles with Simarouba glauca leaf extract
120 ml of freshly prepared Simarouba Glauca leaf extract solution was added to aqueous Copper acetate (50 ml, 0.1 M) solution drop wise with stirring using a magnetic stirrer for 1 hour. The blue colour of the solution first change to green and then dark brown on the addition of plant extract. The brownish black coloured precipitate indicates the formation of CuO nanoparticles. Then this mixture was sonicated for 30 minutes, allowed to settle overnight, filtered, washed with distilled water, dried and annealed using a muffle furnace at 5000C for 2 hours to obtain the product.
Characterization of nano CuO
The phase purity and crystallinity of the prepared material were verified by powder X-ray diffraction technique (XRD) of Bruker AXS D8 Advance model with Cu Kα radiation. The functional groups were identified with Fourier transform infrared (FT-IR) spectrometer of Thermo Nicolet, Avatar 370 model by converting the product into pellet using KBr. The absorption spectra of the powder sample were recorded at room temperature using UV-VISIBLE spectrophotometer of model Cary 5000. Surface morphology of the sample was analyzed by SEM of JEOL Model JSM – 6390LV, and particle size and shape by TEM of JEOL/JEM 2100.
Adsorption study on Nano CuO
Adsorption studies on the surface were performed by batch process. In this process, taking 0.1 g of synthesized CuO and 100 ml 10 mg L-1 Pb2+ solution in a stoppered conical flask and placed on a mechanical shaker at 160 rpm. The rate of adsorption of lead on CuO nanoparticles after desired time intervals [30, 60, 90, 120,150 min. etc], was determined by measuring absorbance using atomic absorption spectroscopy. The experiments were repeated for different concentration, adsorbent dosage and pH for heavy metal ion solutions. The adsorption efficiency (q) and removal percentage are determined as follows:
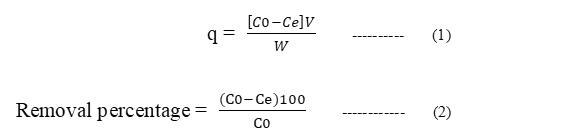
where q is adsorption efficiency of the adsorbent [mg.g-1], W is weight of adsorbent [g], V is volume of solution [L], Co [mgL-1] and Ce [mgL-1] are initial and equilibrium concentration of adsorbate in the solution respectively.
Results and Discussion
The nano CuO prepared in the present study was obtained as black fine powder.
XRD Analysis
The powder XRD pattern of the nano CuO obtained is shown in Fig.1. The XRD pattern of CuO nano structures (Fig.1) is well matched with single phase monoclinic structure and well consistent with the standard data reported by JCPDS card no. [80-1916]. XRD pattern of nano CuO have no impurity peaks indicating the high phase purity of the product obtained. The small crystal size is indicated by broadening of the peaks in XRD spectrum. The average particle size of the prepared CuO nanoparticles were determined by Debye–Scherer’s formula,

where, D is size of the particle (nm), k is a constant (0.94), λ is the wave length of X-rays used (1.5406°A), β is full-width at half maximum (FWHM) of the peak (in radians) and θ is Bragg angle (degree). The average particle size was found to be nearly 18–24 nm.
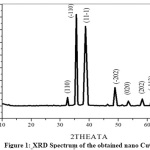 |
Figure 1: XRD Spectrum of the obtained nano CuO Click here to View Figure |
FT-IR Spectral Study
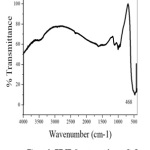 |
Figure 2: FT-IR Spectrum of nano CuO Click here to View Figure |
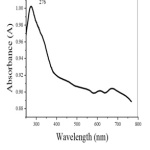 |
Figure 3: UV-Visible Spectrum of nano CuO Click here to View Figure |
Fig.2 shows the FT-IR spectrum of the obtained CuO nano particles. The narrow absorption peak at 468 cm-1 is assigned for Cu-O bond vibration. No other functional groups are present in IR spectrum indicates the purity of the prepared CuO nanoparticle.
UV-VISIBLE spectrum
A strong absorption peak at 276 nm in the UV-Visible spectrum (Fig.3 ) indicates the formation of nano CuO [24].
SEM-EDX Investigation
Fig.4 shows the EDX Spectrum of the prepared nano CuO. The elemental composition is given in Table-1. EDX spectrum shows only peaks corresponding to Cu and O which also verifies the formation of nano CuO in accordance with previous report [9].The SEM image (Fig.5) shows nearly spherical morphology for CuO nanoparticles.
Table 1: Percentage composition of elements from EDX spectrum
|
Element |
Weight % | Atomic % |
|
O |
15.79 |
42.69 |
|
Cu |
84.21 |
57.31 |
| Total | 100 |
100 |
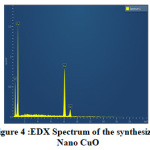 |
Figure 4: EDX Spectrum of the synthesized Click here to View Figure |
 |
Figure 5: SEM image of obtained nano CuO Nano CuO Click here to View Figure |
TEM Investigation
TEM image in Fig. 6 reveals the presence of spherical particles of CuO with uniform morphology having particle size nearly 20nm. Selected area electron diffraction (SAED) in Fig.7 shows the crystalline nature for nano CuO. The diffraction rings in SAED pattern match well with the peaks in XRD spectrum. This also confirms the monoclinic nature of the prepared nano CuO.
Adsorption of lead (II) from solution
Small portion of the Pb (II) solution was withdrawn at regular intervals and absorbance was measured by Atomic absorption spectrometer. It was observed that the absorbance of the solution decreases with increasing time intervals.
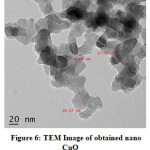 |
Figure 6: TEM Image of obtained nano CuO Click here to View Figure |
 |
Figure 7: SAED pattern of as prepared nano CuO Click here to View Figure |
Effect of Concentration of Pb2+ ions
Effect of adsorbate concentration in the rate of adsorption was determined by varying the concentration of Pb2+ ions from 10 to 40 mgL-1(Figure. 8). It can be seen that rate of adsorption is maximum for 10 mgL-1 and then decreases. At low concentration the ratio between surface active sites and the total metal ions in solution was very high. Then all the ions of metal interacted with nano CuO and are carried away from solution. On increasing the concentration, the mass of metal ion adsorbed per unit mass of adsorbent was low due to high concentration gradient[25].
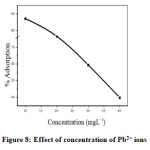 |
Figure 8: Effect of concentration of Pb2+ ions Click here to View Figure |
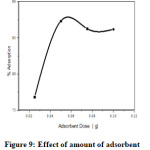 |
Figure 9: Effect of amount of adsorbent Click here to View Figure |
Effect of Amount of nano CuO
Effect of amount of nano CuO on the rate of adsorption was examined by varying from 0.025 to 0.1g/100ml of 10ppm Lead solution (Figure.9). It was observed that the maximum extent of adsorption takes place at 0.05g of nano CuO. But at higher dose (0.075 to 0.1g), no further increase of adsorption takes place due to the fact that the amount of Pb2+ held to the nano CuO and the amount of free Pb2+ in solution remains constant [26, 27].
Effect of pH
The removal ability of adsorbent depends on pH of solution. Dependence of pH on metal adsorption is related to both metal chemistry and ionization state of the sorbent there by affecting the number of binding sites [28, 29]. The surface of metal oxide in aqueous phase consists of hydroxyl groups which depend on pH of solution [28]. Variation of adsorptivity with pH is explained by the behavior of hydrogen ion and counter ion activity in aqueous solution. The hydrogen ion activity decreases up to pH 6 and percentage adsorptivity increases. Above pH 6, adsorptivity decreases since concentration of hydrogen ion is too low and precipitation occurred in strong basic pH [29, 30].
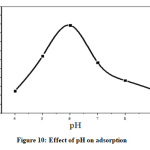 |
Figure 10: Effect of pH on adsorption Click here to View Figure |
Conclusion
Synthesis of pure nano CuO by green route is a simple, environment friendly, non-toxic and low cost using aqueous leaf extract of Simarouba glauca. Metabolites present in the leaf extract of Simarouba glauca can act as bio-reducing agent and also as a capping agent in the synthesis nano CuO. CuO nanoparticles with monoclinic structure and spherical shape with approximately 20 nm size is reported. In the present work, nano copper oxide is used for the adsorption of Pb2+ from solution. Optimum reaction conditions for the 95% removal of Pb2+ on nano copper oxide were experimentally determined and found that lead (II) ion concentration of 10mgL-1, adsorbent dose 0.05g and pH 6. The synthesized nano CuO may be used for the elimination of other inorganic pollutant from industrial waste water.
Acknowledgements
One of the authors (G.Sreekala) is grateful to UGC, New Delhi for providing the financial assistance in the form of FDP. STIC-CUSAT is also acknowledged for instrumental facilities.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- Zhou,Y .M.; Hu, X. C.; Zhang, M; Zhuo. X. F; Niu. J .Y. Ind. Eng. Chem.Res.2013, 52, 876-884
- NSC. Lead poisoning available at, http://www.nsc.org/library/ facts/lead.htm; 1.9.2007.
- Naiya, T.K;, Bhattacharya, A.K;, Das, S. K. J Colloid Interface Sci. 2009, 333,14 – 26
- Rao, R. A. K; Ikram, S. Desalination. 2011,277, 3908.
- Ding, Y.; Jing, D.; Gong, H;, Zhou, L.; Yang, X. Bioresour Technol. 2012, 114,20-25
- Witek-Krowiak ,A; Szafran, R,G; Modelski, S. Desalination. 2011, 265,12634
- Kannan, N.; Sundaram, M. M. Dyes Pigm. 2001, 51, 25-40
- Hu, J.; Chen, G.; I. M. C. L. Journal of Environmental Engineering. 2006, 132,709-715
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M. A.; Reip, P.;Allaker, R. P. Int J Antimicrob Agents. 2009, 33, 587- 590
- Iravani, S. Green Chem. 2011, 13, 2638-2650
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima,T.;Shivashangari, K. S.; Ravikumar,
- Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014, 121,746-750
- Shankar. S. S.; Rai, A.; Ahmad, A.; Sastry, M. Journal of Colloid and Interface Science. 2004, 275, 496–502
- Gardea-Torresdey,J. L.; Parsons, J. G.; Gomez, E. Nano Letters. 2002, 2, 397–401.
- Gardea-Torresdey, J. L.; Gomez, E.;, Peralta-Videa,J. R., Parsons, J. G.; Troiani, H.; Jose-Yacaman, M. Langmuir. 2003, 19, 1357–1361
- Huang,J.; Li,Q.; Sun,D. Nanotechnology. 2007, 18, 105104–105115
- Ankamwar,B.; Damle,C.; Ahmad. A.; Sastry.M. Journal of Nanoscience and Nanotechnology. 2005, 5, 1665–1671
- Shankar,S. S.; Rai, A.; Ankamwar, B.; Singh, A. Ahmad, A.; Sastry,M. Nature Materials. 2004, 3, 482–488,
- Ankamwar, B; Chaudhary, M.;Sastry, M. Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry. 2005, 35, 19– 26
- Shiva Prasad, K.; Patra, A.; Shruthi, G.; Chandan, S. Journal of Nanotechnology. 2017, 2017, 6 pages.
- Sivaraj, R.; Rahman, P. K. S. M.; Rajiv, P.; Narendhran, S.; Venkatesh, R. Spectrochemica Act Part A:Molecular and Biomolecular Spectroscopy. 2014, 129, 255
- Sharanya,V. K.; Gayathiri, K.; Sangeetha, M.; Shyam, P. G.; Gopi, S. K.; Vimalavathini, R.; Kavimani,S. A. Int. J. Pharma Res. and Rev. 2016, 5,32-36
- Ghahi, A. Introduction to pharmacognosy, Ahmadu Bello University press, Ltd. Zaria,Nigeria, 1990, 45-47
- Patil, M. S.; Gaikwad, D. K. J. Pharm. Sci. & Res. 2011,3, 1195-1213
- Wang, H.; Zhang,J .J; Zhu, H.Y.Chen,J.Cryst. Groth. 2002, 244, 88.
- Fu, F. L, Wang, Q, J. Environ. Manage. 2011, 92, 407-418
- Yogesh Kumar, K.; Muralidhara, H. B.; Arthoba, Y. Powder Technol. 2013, 239, 208–216
- Heidari, A; Younesi, H; Mehraban, Z.Chem Eng J. 2009; 153,709
- Mendez, J. R. R; Zepeda, R. M; Ramos, E. L; Flores, P. E. D; Shirai, K. J Hazard Mater. 2009, 162, 503–11
- Ngomsik, A. F; Bee; Siaugue,J. M.; Talbot, D.; Cabuil, V.; Cote, G. J Hazard Mater. 2009, 166, 1043-9
- Hu, J. Chen, G.; Lo, I. M. C. Water Research. 2005, 39, 4528-4536
- Kim, M. S.; Hong, S. C.; Chung, J. G. Environ. Eng. Res. 2005, 10, 45-53
- Kosa, S. A.; Zhrani, G. A.; Salam, M. A. Chem. Eng. J. 2012, 181−182, 159−168

This work is licensed under a Creative Commons Attribution 4.0 International License.









