Titanium dioxide as a Catalyst for Photodegradation of Various Concentrations of Methyl Orange and Methyl Red dyes using Hg Vapour Lamp with Constant pH
A. Kistan1,2 , V. Kanchana3, L. Sakayasheela2, J. Sumathi2, A. Premkumar2, A. Selvam2 and Thaminum Ansari A4
, V. Kanchana3, L. Sakayasheela2, J. Sumathi2, A. Premkumar2, A. Selvam2 and Thaminum Ansari A4
1Research and Development Centre, Bharathiar University at Coimbatore, India.
2Panimalar Institute of Technology, Chennai-123, India.
3Sree Sastha Institute of Engineering and Technology, Chennai, India.
4P.G. Research department Muthurangam Government Arts College, Vellore.
Corresponding Author E-mail: vishmikrish@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340250
Article Received on : January 01, 2017
Article Accepted on : February 10, 2018
The photo-catalytic activity of TiO2 catalysis is very much useful to removal of color from dye industry effluent from tanneries, textile industry, and Ink factory. The Novelty of this study, we have found on a very simple method when to compare the photo catalytic activity of Titanium dioxide in different Concentration of two different Organic dyes (Organic dyes of Methyl Orange and Methyl Red) which were not compared in the past studies. This method can be carried out in a normal chemical lab and easy way with limited use of chemicals and simple lab equipment’s. Dye solutions having pigments with high photo catalytic activity lose their color within several minutes of UV-Visible radiation exposure (optical windows 160W (Hg lamp medium pressure)) where as, dye solutions having UV-stable TiO2 would not degrade within several hours of radiation exposure. For both dyes (Methyl Orange and Methyl Red) the color started fading quickly when the light radiation was passed continuously for a long time (15 to 120 minutes). It was also found that concentration of both dyes decreased, with time or the degradation increased. The effect of varying the dye concentration increases with degradation rate decreases. The rate constant value undergoes some marginal decreases at pH = 4.2 when the Methyl Orange dye concentration was increased. The degradation Measurements were carried out using SHIMADZU UV-Visible 1601 spectrometer in the range of wave length from 200nm to 700 nm aid of Glass cell (made of Quartz) with 10mm optical path length.
KEYWORDS:Organic Dyes; Hg lamp; pH; Methyl Orange (MO); Methyl Red (MR); Degradation
Download this article as:| Copy the following to cite this article: Kistan A, Kanchana V, Sakayasheela L, Sumathi J, Premkumar A, Selvam A, Ansari T. A. Titanium dioxide as a Catalyst for Photodegradation of Various Concentrations of Methyl Orange and Methyl Red dyes using Hg Vapour Lamp with Constant pH. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Kistan A, Kanchana V, Sakayasheela L, Sumathi J, Premkumar A, Selvam A, Ansari T. A. Titanium dioxide as a Catalyst for Photodegradation of Various Concentrations of Methyl Orange and Methyl Red dyes using Hg Vapour Lamp with Constant pH. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=43991 |
Introduction
Water Pollution by Tanneries, Textile and dye Industries
More than thirty thousand trading dyes founded. According to more than eighty thousand various chemical structures are utilized in industries such as textile, tanneries, food, pharmaceutical, paper and ink industries. And sewage water from these industries normally discharged in water bodies without recylcing3. The effluent containing these types of impurities are normally removed by physical, oxidative or common method called as sludge process. Above mentioned process has a various draw backs and its create secondary pollution due to chemicals which were used for the removal process and its accumulated in species creates bio-resistant in the environment. Both textile and tanneries are very highest (more 50%) user of dyes. Utilizing dyes in wide range of supporting reagents for different coloring skins, dyeing, cleaning leathers, printing specially for finishing procedure. Mill effluents, Fiber class or irrespective of dyes are extremely colored by unabsorbed dyes were varied due to the dye-fiber system.
In textile industry Azo- dyes are normally used and create the various environmental issues. In general waste water produced from various industrials includes tanneries, food and pharmaceuticals, and ink industries are utilizing dyes for various purposes, which requires proper treatments afore it is released to the surrounding. In addition, effluent streams released from tanneries and textile industry consists pollutants like toxic or carcinogenic substances along with their products. Recently, removal process is more concentrated not only to elucidate the environment impact but also suggest that used dyes can be recycled.
Treatment of Polluted Water
From the environmental perspective, the elimination of synthetic dyes is one of the great concerns, because degradation of dye and dye by-products are also may be carcinogenic so treatment cannot fully dependents upon the biodegradation alone. Henceforth, decolonization of dye house effluent by elimination of dyes has become a very essential need in waste water treatments, tanneries and textile industry2. The environment concerns about use of dye in effluent ongoing problem for dye stuff productions, dyers and water industries. The problem of color in waste water is common in many Tanneries and dyes houses. Hence, significant increase in standards of stringent color is enforced by common bodies to decrease the amount of effluent and water courses. Many methods are employed to treat waste water containing organic dyes and pigment such as adsorption, sedimentation, flocculation, floatation, reverse osmosis, fractionation etc., but the treatment of dye containing waste water containing dye by the above conventional methods has various limitation due to the significant increase in the refractory materials originated in waste water effluents, complications in the elimination of color and expensiveness. Elimination of organic non-biodegradable chemicals is a main problem. Dyes are main type of synthetic organic compounds utilized in textile industry as well as its also common industrial pollutants, due to modern dyes stability, management of biological treatments techniques for industrial waste water are ineffective resulting often in a colored release from the treatment techniques 5-8. The above problems could be overcome by the ‘photo catalytic method’ which is widely employed in complete degradation process. The direct photo catalytic reaction by using semiconductor powders has been shown to effectively degrade many kinds of pollutants.
Photo Degradation
Photo degradation is nothing but degradation of molecules caused by the absorption of radiation (UV or visible) i.e., because of absorption the dissociation of molecules into smaller pieces. There are also irreversible structural changes such as the structural changes of proteins, and addition of other atoms or molecules takes place. One of the Photo degradation reaction is photo-oxidation10. This type of Photo degradation is used by some drinking water facilities to destroy pollutants and also to kill the microorganisms Photo degradation in the environment is part of the process by which ambergris evolves from its fatty precursor. Photosensitized degradation of colored contamination in waste water on surface of the semiconductor is current importance10-14. Although semi-conducting TiO2 in presence of UV radiation has become the a standard or point of references against which things may be compared photo-catalyst for the degradation of myriad or a water bound.15-18 pollutants as documental in the literature, the important drawback of TiO2 semiconductor is that it absorbs a small portion of solar spectrum in the UV region (band gap energy of TiO2 is 3.0 to 3.2eV). hence the photo-catalytic process has the disadvantage that it cannot harvests maximum solar energy by using the visible light. A large number of dyes stuff material so with intense color and toxicity introduced into the aquatic systems, are resistant to degradation. More recently the degradation of colored ones in environmental protection studies has gained importance. Azo dyes are typical class of organic pigments and they are represented as toxic compounds. Method of decolorizing dye effluents has been gaining attention. An inherent advantage of heterogeneous or homogenous photo catalysis emerges when solar irradiation is used as a photon source19.
Titanium DioxideAs Photo Catalyst
TiO2 semiconductor is a very good pollutants removal and also it acts as a Photo catalyst for the elimination of pollutants from air and water due to its low cost, chemical stability, no toxicity, high photocatalytic reactivity and insolubility. A further vital advantage is the fact that the process can be powered by solar energy20, 21, thus reducing significantly the electrical power requirements and costs for the removal process. It should be noted that with visible light, the Photo degradation processes by different routes are available. Specially involving electron transfer from the excited state of the dye molecules adsorbed on the TiO2 surface into the conduction band of TiO2 are possible but such process is less efficient than those occurring with UV light4.For the photocatalytic oxidation ((TiO2/UV)), the semiconductor absorbs UV light and generated hydroxide Radicals mainly from the absorbed water and hydroxide ions. Process of this mechanism of the TiO2/UV degradation has been described using the band-gap model22. It is well established that by irradiation of an aqueous TiO2 suspension with light energy greater than the band gap energy of the semiconductor, conduction band electrons and valence band holes are generated23. These will act as strong oxidizing agents and adsorbed organic molecules or located close to the surface catalyst, thus leading to their complete degradation into small inorganic species.
TiO2 + hv → TiO2 (h+ + e–) (hv≥Eg = 3.2eV; λ = 390nm)
O2 + e– → O2-
H2O + h+ → H+ + OH*
OH* + RH → H2O +R*
R + O2 → ROO* → → CO2 + Other products
Earlier work of Photo Degradation
The photo-assisted degradation of erythrosine and Rhodamine B has been investigated in an aqueous TiO2 dispersion under irradiation by using visible light24. Wang investigated25 the photocatalytic degradation of commercial dyes including MO using TiO2 suspension under solar light. The possibility of using solar irradiation for detoxification of MO as a model compound was also explored by other workers26. Wang27 prepared and investigated the photocatalytic activity of ZnO/TiO2/SnO2 mixture for decolonization of MO under UV light and found that this mixture was photo catalytically more active than TiO2 and SnO2 but slightly less active ZnO28. In recent years, the Photo catalyzed oxidation kinetics of acid blue-40 at different pH Values by TiO2/UV were studied32. It is found that pH =3, photocatalytic Oxidation kinetics of Acid blue40 follows the Langmuir Hinshelwood Model with reaction rate constant (k) of 0.0074m/min. It is found that ferrous ion does not have significant influence on the reaction while H2O2 could retard the reaction when its concentration is higher than 0. 01nm.Photo degradation of series of dyes (Rhodamine B, Orange II, Alizarin red and Eosin) in the presence of TiO2 particles under visible light irradiation led to formation of H2O2. H2O2 was detected because its formation rate was greater than decomposition rate33. various parameters such the effects of as catalyst loading, pH and initial concentration of the dye has been examined35.
The degradation was strongly enhanced in the presence of H2O2, (NH4)2S2O8 and KBrO3 as electron acceptors 35. The photocatalytic decolorization of reactive Blue19 (RB-19)34-38. In aqueous solutions containing TiO2 or ZnO as catalyst and concluded that ZnO is more efficient catalyst that TiO2 in the color removal of RB of RB-1939. Generally degradation rates were compared with disappearance and elimination rates. In this photo catalytically Degradation of Azo dye, the disappearance proceeds through both Redox reactions41.Photo catalytically degradation of Methylene blue a cationic dye and procion red, an anionic dye has been examined under TiO2 also pH dependent because of its amphoteric nature. It has been found that pH -9.5 and pH -3.5 are suitable for Methylene blue and procion red respectively42. The degradation of biological dyes such as Methylene blue and Malachite green 7 was carried out with Nano TiO2 which have a particle size of 16-17nm. The absorption peak of Methylene blue decreases with Photo catalyst time43.
Desensitization, a method according for degradation of colorants in actinic radiation well-lighted dye changed TiO2 dispersion, might even be possible for degradation of colorless lake water pollutants44,45. Recently a number and researchers have restricted heterogeneous photocatalytic decomposition of dye within the presence of ultraviolet illumination or visible light46.
TiO2 + hv → h+(VB) + (CB) e–
h+(VB) + OH–(water) → OH* (ads)
H2O(ads) + h+(VB) → OH* (ads) + H* (ads)
Dye +OH* (ads) → Degradation of the dye.
Desensitization, a method according for degradation of colorants in actinic radiation well-lighted dye changed TiO2 dispersion, might even be possible for degradation of colorless lake water pollutants44,45. Recently a number and researchers have restricted heterogeneous photocatalytic decomposition of dye within the presence of ultraviolet illumination or visible light46. Aerophilous method has been additionally accustomed decolorize and mineralize several styles of group dyes in a very bench scale by exploitation each artificial irradiation47,48 and star technology, industrial dyes in TiO2 suspension underneath star lightweight however the set of equipment employed by wang49 were regardful and concentration of the dye resolution was totally different in comparison to the current work.
Novelty of in this present study: we have found on a very simple method to compare the photocatalytic activity of TiO2 for different Concentration of two different Organic dyes (Methyl Orange and Methyl Red) which were not compared in the past studies. This experiment was done in a normal chemical lab and easy way with limited use of chemicals and simple lab equipment’s. For both dyes (Methyl Orange and Methyl Red dyes) the color started fading quickly when the light radiation was passed continuously for a long time (120 minutes).
It has been observed that concentration of both dyes decreased, with time or the degradation increased. The effect of varying the dye concentration increases with degradation rate decreases. The rate constant value undergoes marginal decreases at pH = 4.2 when the Methyl Orange dye concentration was increased. The rate constant value undergoes a marginal decrease at pH= 4.5 when the Methyl Orange dye concentration was increased. From these comparisons it has been clearly observed that degradation of Methyl Red Organic dye (MR) dye slightly faster than degradation of Methyl Orange dye (MO).
The present work deals with photocatalytic (TiO2/hv) degradation for color removal from solution containing acid-base indicator (Methyl Orange) and alkyl Red (Methyl Red) dyes. In this study Mercury (Hg) vapour lamps used as irradiation sources. Utilization of daylight because the ultraviolet radiation energy supply is useful from ecological purpose. The dependence of dye icon oxidization rate on the subsequent parameters: Initial dye concentration, irradiation time, irradiation intensity was investigated additionally.
Scope of the Investigation
The removal of artificial dyes in effluent water from workplace industries, dye industries, textile industries etc., is of nice concern since a number of the dyes and its degradation products are harmful to human life. Therefore degradation of dyes becomes a crucial facet within the treatment of waste water. Several strategies are used for the dye degradation method however the ‘photocatalytic degradation method’ as wide used for the entire degradation method chiefly thanks to economic practicability and also the easier nature of the technique concerned. Much attention has been centered for the past 2 decades on the photocatalytic degradation of organic pollutants mediate by TiO2 particles in liquid dispersion irradiated by UV radiation and visible light32.
 |
Scheme 1 Click here to View scheme |
The big proof that has been collected and suggests that this technique is beyond any doubt with a possible and effective approach towards the degradation of harmful organic pollutants in waste water and towards the purification of beverage. Hence TiO2 is one the normally used environmental icon catalyst for the reaction of liquid organic pollutants and TiO2 catalyst exhibits smart activity for the icon reaction or chemical group dyes31.
Visible light pathway
The mechanism of photocatalytic degradation of dye by visible light has different from that of UV irradiation. In this process, the dye is excited by the presence visible light and not by the presence of semiconductor TiO2 is mixed with Methyl Orange and Methyl Red solution and magnetically stirred under visible light irradiation (λmax = 365nm). The TiO2 particles are isolated from the solution and the change in the absorbance of Organic Methyl Orange and Organic Methyl Red dyes are monitored spectrometric ally. The rate constant for the degradation kinetics is determined for
Effect of dye concentration
% of degradation is evaluated.
Experimental method
Preparation of Methyl Orange solution
Preparation of Methyl Orange solution
A stock solution (1 X 10-3M) of Methyl Orange was prepared by dissolving 0.032g of Methyl Orange powder (A.R. Qualigens) in 100ml of deminineralised water.
Preparation of Methyl Red solution
A stock solution (1 X 10-3M) of Methyl Red prepared by dissolving 0.28g of Methyl Red powder (A.R. Qualigens) in 5ml and 95ml of demineralised water.
Photo- reactor and light source
The Photo- reactor used for the degradation method could be a cylindrical cell with optical windows 160w (Hg lamp medium pressure). The sunshine supply is Hg lamp medium pressure actinic ray culminated by lens. The setup was lined employing a cardboard box to make sure darkness throughout the passage of sunshine from the sunshine supply.
Degradation Experiments
The aqueous TiO2 suspension was prepared by the addition of 30mg of Titanium dioxide (A.R.Qualigens) to a 50ml of aqueous dye solution, whose concentration is changed between 1 X 10-5N to 1 X 10-4N. The suspension was magnetically stirred with irradiation within the dark for 1hr to confirm the institution of associate degree adsorption/desorption equilibrium. The dispersions were unbroken underneath constant air equilibrated condition before and through the passage irradiation. At regular time intervals, 5ml of aliquots were collected and centrifuged to get rid of the TiO2 particulate. The unvaried solutions were collected and their sorption measured victimization SHIMADZU UV-Visible 1601 spectroscope.
Degradation Kinetics
The decrease in the absorbance of the Methyl Orange and Methyl red dye solutions are monitored by UV-Visible spectrometer at 460nm and 550nm respectively. The decrease in absorbance is noted as function of time in minutes. The visible spectrum is recorded in the region of 300nm to 700nm both prior and after irradiation.
Absorbance Measurement
The absorbance of dye goes to scientific method. Solutions before and after degradation were measured at completely. Degradation time and different irradiation sources Measurements were meted out victimization SHIMADZU UV-Visible 1601 spectroscope within the gauge boson energy vary of wave length three hundred to 700nm aid of Glass cell(made of Quartz) with 10mm optical path length. The degradation percentage was calculated from the following equation:
![]()
Where At is that the absorbance once time interval t and A0 is that the absorbance of initial concentration of dye before degradation.
Result and discussion
Study of Photo Degradation
The degradation of the dye in aqueous solution occurs when it is irradiated with visible light (Fig. 1.c and 2.c) The photocatalytic degradation of Organic dyes of Methyl Orange and Methyl Red with Titanium dioxide catalyst obeys the first order kinetics at all dye concentration and rate expression is given by the equation.
![]()
Where k1 is the first order rate constant. The dye is adsorbed on to TiO2surface and the adsorption-desorption equilibrium is reached within 30 minutes. The equilibrium constant of the dye solution is determined after this period and it is taken as the initial dye concentration for the analysis of kinetic studies. Integrating the above equation with the limit of C = C0 and t = 0 with C0 being the equilibrium concentration of the bulk solution
![]()
where C0 is the equilibrium concentration of dye at the initial and C is the concentration at time t and k1constant.. The plot of log (a – x) against Time follows the first order kinetics, and from this plot the rate constant is calculated.
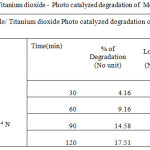 |
Table 1a: Kinetic plot of Visible/ Titanium dioxide – Photo catalyzed degradation of Methyl Orange dye |
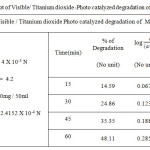 |
Table 1b: Kinetic plot of Visible/ Titanium dioxide-Photo catalyzed degradation of Methyl Orange dye Click here to View figure |
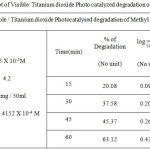 |
Table 1c: Kinetic plot of Visible/ Titanium dioxide Photo catalyzed degradation of Methyl Orange dye Click here to View figure |
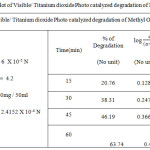 |
Table 1d: Kinetic plot of Visible/ Titanium dioxidePhoto catalyzed degradation of Methyl Orange dye Click here to View figure |
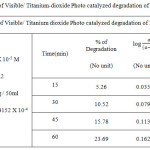 |
Table 2.a: Kinetic plot of Visible/ Titanium-dioxidePhoto catalyzed degradation of Methyl Red dye Click here to View figure |
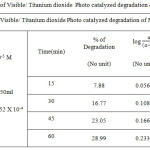 |
Table 2.b: Kinetic plot of Visible/ Titanium dioxide Photo catalyzed degradation of Organic Methyl Red Click here to View figure |
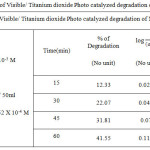 |
Table 2.c: Kinetic plot of Visible/ Titanium dioxidePhoto catalyzed degradation of Methyl Red dye Click here to View figure |
 |
Graph 1: Kinetic graphs of Visible / Titanium dioxide Photo Catalyzed degradation of Methyl Orange dye Click here to View figure |
 |
Graph 2: Kinetic graphs of Visible / Titanium dioxide Photo Catalyzed degradation of Methyl Red dye (MR) Click here to View figure |
Effect of Concentration in dye
The concentration of pollutants is a different type of parameter in the wastewater treatment. The effect of various initial dye concentrations on the photocatalytic decolorization and degradation has been investigated with dye solution. The degradation rate constant for the Methyl Orange dye and Methyl Red dye under same pH and concentration values are shown in the table.3 with respect to pH = 4.2, Temperature = 270C and λmax = 460nm and pH = 4.5, Temperature = 270C and λmax = 520nm.
Table 3: The rate of color degradation of Methyl Orange and Methyl Red dyes
|
[CATALYST] (mg)/50ml |
Degradation of Methyl |
Degradation of Methyl Red |
||
|
Orange dye Rate constant |
dye Rate constant X 10-4 |
|||
|
S.N0 |
[Dye] (M) |
X 10-4 Sec-1 |
Sec-1 |
|
|
(pH at 4.2) |
(pH at 4.5) |
|||
|
1 |
1 X 10-5 |
30 |
2.4152 |
– |
|
2 |
4 X 10-5 |
30 |
1.2748 |
9.6282 |
|
3 |
5 X 10-5 |
30 |
1.2507 |
8.0551 |
|
4 |
6 X 10-5 |
30 |
0.8418 |
7.6653 |
It determined that from higher than the Table.1, Table 2, Table 3, Graph1 and Graph2 pair of shown the degradation rate constant decreases perpetually with [dye] concentration, degradation of different dyes. [39,40,41 Thus, the time taken for the entire degradation of the dye is most for the dye of upper concentration and it’s minimum for the dye of lower concentration. Once the concentration will increase, the number of dye absorbed on the chemical process surface is increase. It also affects the chemical activity of Titanium dioxide. The rise in dye concentration conjointly decreases the trail length of gauge boson getting in the dye solutions. At high dye concentration, a big quantity of lightweight ultraviolet illumination ultra violot actinic radiation light is also absorbed by the dye molecule instead of the catalyst and this might cut back the chemical process potency.
Visible Light Pathway
The mechanism of photocatalytic degradation of dye by visible light has been different from that of UV irradiation. In this process, the dye is excited by the visible light and not influenced by the semiconductor Titanium dioxide
Mechanism
Initial of Photocatalytic reaction
Dye + hv → Dye* (initiation)
Dye + TiO2 → Dye*+ TiO2(e)
TiO2(e) + O2 → TiO2+ O2–4.TiO2(e) + O2–+ 2H+ → H2O2
H2O2 + TiO2(e) → OH* + OH–
Dye*+ O2* (or O2-or OH*) → Peroxalates or hydroxylated intermediates
Subsequently, Titanium dioxide plays the vital role of an electron and dye cation radials. The processes is described in equations from the above 1 to 5. And also this is an example for electron transfer, generation of peroxide radical anion, H2O2 and OH* radical formation have been demonstrated earlier. In this present investigation the degradation of Methyl Orange and Methyl red with TiO2 catalyst is irradiated with visible light in the range of λmax= 365nm.
Photo degradation process of dyes in presnece of visible light
The photolysis of the dye in aqueous solution is carried out by varying the following parameters;
Concentration of the dyes. 2. pH, and the catalyst concentration is kept constant in all experiments.
The solution were irradiated with visible light (λmax = 365nm) near UV light. The absorption peak (λmax for MO is 460nm, and λmax for MR is 520nm) corresponding to the dye diminished and finally disappeared during photolysis, indicating the complete degradation of Methyl Orange. new bands not appeared in the visible region, corresponding to aromatic moieties and other similar intermediates. In the absence of UV light no significant degradation occurred prior to irradiation. Since the photo degradation of dye occurs predominantly on Titanium dioxide surface. The absorption of dyes molecule from aqueous solution on to Titanium dioxide surface is very important. The extent of adsorption of dye at various concentrations in blank solution after the adsorption process equilibrium had been reached i.e. prior to UV irradiation. The absorbance of the solution prior to irradiation provides the initial concentration (C0).
Photocatalytic degradation mechanisms
The complete assumption of dye catalyzed abasement advance that blind bandage holes (h+) and advice bandage electron(electrons-CB) are produced if aqueous TiO2 abeyance is apparent with ablaze activity > than (Eg = 3.2eV) its bandage gap energy. The photon produced electrons can abatement the dye or acknowledge with electron can be abatement the dye or accompany with electron acceptors includes O2 captivated on the Ti(II) – apparent or attenuated in baptize abbreviating to superoxide abolitionist anion O2-. Photo produced holes can burn the amoebic atom to anatomy R*, or added awful oxidant breed (Peroxide radicals). They are appear to be amenable for the heterogeneous. TiO2 photo atomization of amoebic substrate as dyes. Based on this adapted action in the semiconductor exoteric creates the abasement of dyes can be represented as follows.
TiO2 + hv (UV) → TiO2 (electron (CB) + h+(VB))
TiO2 ( h+(VB)) + H2O → TiO2 + H+ + OH–
TiO2 (h+(VB)) + OH– → TiO2 + OH*
TiO2 (electron (CB)) + O2 → TiO2 + O2–
O2-+ H+ → HO2*
Dye+ HO2* → Degradation products
Resultant OH* radical, getting a actual able acerbic abettor (standard redox abeyant +2.8V) can burn a lot of the dye. Substrate not acknowledging against hydroxyl radicals are base employing TiO2 photochemical reaction catalysis with ante of adulteration awful afflicted by the semiconductor blind bandage bend position.
Conclusion
For both the Organic dyes (MO and MR) the blush started crumbling bound while the ablaze radiation was anesthetized continuously for a continued time (2hrs).
It was as well beginning that absorption of both dyes decreased, with time or the abasement increased.
The aftereffect of capricious the dye absorption increases with abasement amount decreases.
The amount connected amount undergoes a bordering abatement (from 2.4152 X 10-4 Sec-1to 0.8418 X 10-4 Sec-1) at pH = 4.2 if the Methyl Orange dye absorption was adapted from 1 X 10-5 to 6 X 10-5 N.
The amount connected amount undergoes a bordering abatement (from 4.6181 X 10-4 Sec-1to 2.7046X 10-5 Sec-1) at pH = 4.5 if the Methyl Orange dye absorption was adapted from 1 X 10-5 to 6 X 10-4 N.
From this analysis it has been empiric that abasement of Organic Methyl Red dye (MR) hardly faster than a basement of Methyl Orange dye (MO).
It has been acclaimed that the abasement of all amoebic dyes blush could be removed by this method.
It was clear that this method applicable for removal color from effluent coming from tanneries, dye industries, Ink industries.
Reference
- Fu, G.; and. Allen, H.E .;Water Res. 1992, 26, 225.
CrossRef - Kannan, N; and MeenakshiSundram,M.; dyes pigments. 2001,25,51.
- Chen. L,; Water Res.,2000,34, 974-982.
CrossRef - Gonculves. M.; Pinto. E,; Nikeonye P,; and Oliveria- Campus. Dyes and Pigments, 2005, 64, 135-139.
- Daton. J.S,; Janes N.G.; Nicholso J. A.; Halman K. R.; Allen. G.C.; Envir. Pollut. 2001, 32, 415-422.
- Malato S,; Marc. G.; Palmisano L.; Pazzi M.; Pramauro. E.; 2009, 49(10),1223-1230.
- Tanaka. K.; Reddy. KSN.; Appl. Catal. B: Environ.2002, 39,305-310.
CrossRef - Konstantinou. I.; Albanis. TA.; Appl. Catal. B: Environ. 2004, 49, 1-14.
CrossRef - International Union of Pure and applied chemistry(IPUAC), 1996. 12, 2223-2286.
- Hoffmann, M.R.; Martin, S.C. ; Choi,W.; Bahnemann, D.W.; Chem. Rev.1995, 95,69.
CrossRef - Stafford,U.; Gray, K.A.; Kamat, P.V.;Chem. Rev.,1996, 3, 77.
- Peral. J.; Domenech, X.;. Ollis. D.F.; J. Chem, Tech. Biotech., 1997, 70, 177.
- Fujshima,A.; Rao. T.N.; Trya. D.A.; J Photo chem. Photobiol c; Photochem Rev.2000, 1, 1.
- Hidaka.H.; Zhao. J.;.Pellzzetti, E.; Serpone, N.; J. Phychem., 1992, 96,2226.
- Sahate, J.; Anderson,M.A.; Kikkawa, H.;.Edwards, M,; Hill C.G.; Jr. J.Catal, 1991, 127,167.
- Fox, M.A.;Dulay, M.T .;Chem. Rev., 1993,93, 341.
CrossRef - Muszkat.L.; Bir, L.; Feigelson, L .;J. Photo- chem. A: chem, 1995, 87,85.
- Stafford, U.; Gray, K.A.;.Kamat, P.V.; Res. Chem. Intermed., 1997,23,355.
CrossRef - Martin. S.C.; Choi. W.; Bahnemann. D.W.; Chem, Rev.1995, 95,69.
- Saquib. M.; and Munner. M.; Dys and pigments, 2002,53,237-249.
- Augugliaro V.; Baiochi. C.; Chemosphere, 2002,49,1223-1230.
CrossRef - Bukelmann. D.; and Shugui. D.; Springer- Verlag, Berlin, 1992, 16, 253-256
- Haffman. M.R.; etal.,chem Rev., 1995, 95,69-96.’
- Zhang. F.; Zhao.J.; Zang., L.; Shen. T.; Catal. A: chem. 1997, 120, 134-138.
- Wang, Y.; Wat. Res. 2000, 34,990.
CrossRef - Al- Qaradawi, S.; Salman. S.R., J. Photochem. Photobiol. A: Chem. 2002,148, 161.
- Wang, C.; Xu, B.; Wang.;J. Zhao. Chemi, 2005, 178,3500.
- Chen. J.; Liu. M., Zhang. J.; Ying. X.; Jin, L.; J. Environ. Manage.2004, 70, 43.
CrossRef - Hoffman. M.R.; Mrtin. S.T.; Chem. Rev., 1995, 95,69-96.
- Hung. C.H.; et al. Water Sci. Tech., 2001, 43(2)313-320.
CrossRef - Tanaka. K.; Padempole, K.;and Hisanaga. T.; 2000, 34(1),327-333.
- Tang W.Z.; and An, H.; Chemosphere . 1995,31,4171.
CrossRef - We, J.; Liu G.;and Zhao. J.; J.Phys. Chem B. 1999, 103,4862.
- VinodGopal, K.; Kamat, Envion . P.V.;.Sci. Technol.1995, 29,841
- Neppolian, B.; Choi, H. C.; Sakthivel. S,; Arabindo. B.;Murugesan, V.; J. Hazzard. Mater. 2002 89,303.
- Lathashree, S.; Nageswara, R.; Shivasankar, B,; Sadassivaam, V.; Rengarai K.;.J. Mole. Catal A: Chem. 2004, 223,101.
- Lizama.C.; Freer,. J.; Baeza. J.; Mansilla. H.D.; Catal. Today.2002, 76,235.
CrossRef - Akyol,A.; Yatmaz. H.C.; Bayaramoglu. M.; Environ, 2004, 54,19.
- Daneswar. N.; Salari. D.; Khataee. A.R.; Chem. 2003, 157,111.
- Sakthivel, S.; Neppolian,B.; Shankar. B.V.; Arabindoo. B.; Palanichamy, M.; Murugesan,V.;Sol Ener. Mater, Sol. Cells 2003, 77,68.
- Vindgopal. K.; Kamat. P.V.; J.Phys. Chem. 1992, 96,5053.
- Hasnat, M.A.; Siddquey. J.A.; and Nuruddin. A.; Dyes and pigments. 2005, 66,185.
CrossRef - Jifan,H.; Zhengai. S.; and Hingliang, L.;GongnengCailiao. 2002, 34,336.
- Chatterjee. D,; Mahat. A.; Appl. CatalB:Environ. 2001, 32(2),119-125.
CrossRef - Chatterjee. D.; Bull. Catal, SOC. India 2004,32,56-58.
- Olivira- Campose A.; Peter O.N.; Poulios, L.; Situation and Perspectives for the European Union 6-10 May 2003, Porto, Portugal. P. 1-6.
- Goncalve, M. S,; Oliverira. T.; Campus. A.M.F.; etal. Chemosphere, 1999, 39,781-786.
- Kiriakidou, F,; Kondarides. Dl.;Very Kios, XE.; Catal. Toaday. 1999, 54,119-130.
CrossRef - Wang G.; Liao. C.; Wa. F.; Chemosphere. 2000, 42,379-387.
CrossRef - Tanaka,K.; Perdernpole. K.;and Hisanaga. T.; Water. Res. 2000, 34,327.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









