Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5245
Peer-review started: January 30, 2021
First decision: May 6, 2021
Revised: May 10, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: July 6, 2021
Hereditary spherocytosis (HS) is a common type of hemolytic anemia caused by a red cell membrane disorder. HS type 1 (HS1) is mostly caused by mutations in ankyrin (ANK1). Newborns with HS1 usually only exhibit anemia and mild jaundice. We herein report a case of HS1 and discuss its clinical characteristics.
A 2-d-old male full-term newborn was admitted to our hospital with severe, intractable neonatal jaundice. Laboratory investigations showed hemolytic anemia and hyperbilirubinemia and excluded immune-mediated hemolysis. The patient underwent two exchange transfusions and one plasmapheresis resulting in significantly reduced serum bilirubin. Hematologic analyses and genomic DNA sequencing studies were performed. The trio clinical exome sequencing revealed a de novo null heterozygous mutation in the patient's ANK1 gene: c.841C > T(p.Arg281Ter). This mutation results in the premature termination of the ANK1 protein.
Our case demonstrates that genetic analysis can be an essential method for diagnosing HS when a newborn has severe hyperbilirubinemia.
Core Tip: Hereditary spherocytosis (HS) is a common type of hemolytic anemia caused by red cell membrane disorder. HS type 1 (HS1) typically results from mutations in ankyrin (ANK1). Newborns with HS1 usually only exhibit anemia and mild jaundice. This paper reports on a Chinese neonate who developed severe, intractable neonatal jaundice unrelated to immune-mediated hemolysis. The patient underwent two exchange transfusions and one plasmapheresis, which significantly reduced his extreme hyperbilirubinemia. Using trio clinical exome sequencing, we identified a de novo null heterozygous mutation in the patient's ANK1 gene: c.841C > T(p.Arg281Ter), which resulted in the premature termination of ANK1 protein.
- Citation: Wang JF, Ma L, Gong XH, Cai C, Sun JJ. Severe hyperbilirubinemia in a neonate with hereditary spherocytosis due to a de novo ankyrin mutation: A case report. World J Clin Cases 2021; 9(19): 5245-5251
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5245.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5245
Hereditary spherocytosis (HS) is the most common monogenic hemolytic anemia disease and is characterized by spherical-shaped erythrocytes in patients' peripheral blood. Approximately 75% of HS cases are inherited in an autosomal dominant manner[1]. The clinical manifestations of HS vary widely, ranging from almost asymptomatic to dependence on blood transfusions or severe life-threatening anemia. HS occurs worldwide, particularly in Northern Europe and North America, where the incidence is 1 in 1000 to 2000 births[2]. In China, the epidemiology of HS is poorly understood. A systematic review from China estimated that the prevalence was 1.27 cases per 100000 people in males and 1.49 cases per 100000 people in females between January 1987 and December 2013[3]. However, only one-third of children are diagnosed with HS in the first year of life[4], and HS is rarely diagnosed in the neonatal period. HS is thought to have a high underdiagnosis rate due to limited clinical recognition during the neonatal period. The clinical manifestations of HS include anemia, jaundice, and splenomegaly. More than two-thirds of neonates with HS have near-normal hemoglobin (Hb) values at birth but then develop a dramatic decrease in Hb levels during the first weeks of life[5]. Physiological jaundice is the most common presenting feature of HS in neonates without any treatments[6], and splenomegaly is rarely detected[5]. The characteristic spherocytes are observed less often in the blood smear of neonates[7].
ANK1 mutations (approximately 50%) are the most common cause of HS type 1 (HS1)[8]. The functional defects of ankyrin protein, located in the red blood cell membrane, lead to the loss of membrane cohesion, a loss of surface area, and an increase in the number of peripheral blood cells[9]. ANK1 mutations are associated with both dominant and recessive HS and are frequently mutated de novo[10]. Using exome sequencing to detect pathogenic mutations in ANK1 and other genes would allow an early HS diagnosis.
This report describes a unique case of HS1 in a Chinese neonate with extreme hyperbilirubinemia and anemia, which was treated with two exchange transfusions and one plasmapheresis. Using the trio clinical exome sequencing method, we identified a de novo ANK1 null mutant causing HS1. This report will contribute to the understanding and diagnosis of HS in neonates.
The patient was a 2-d-old male newborn admitted to the Department of Neonatology due to severe jaundice.
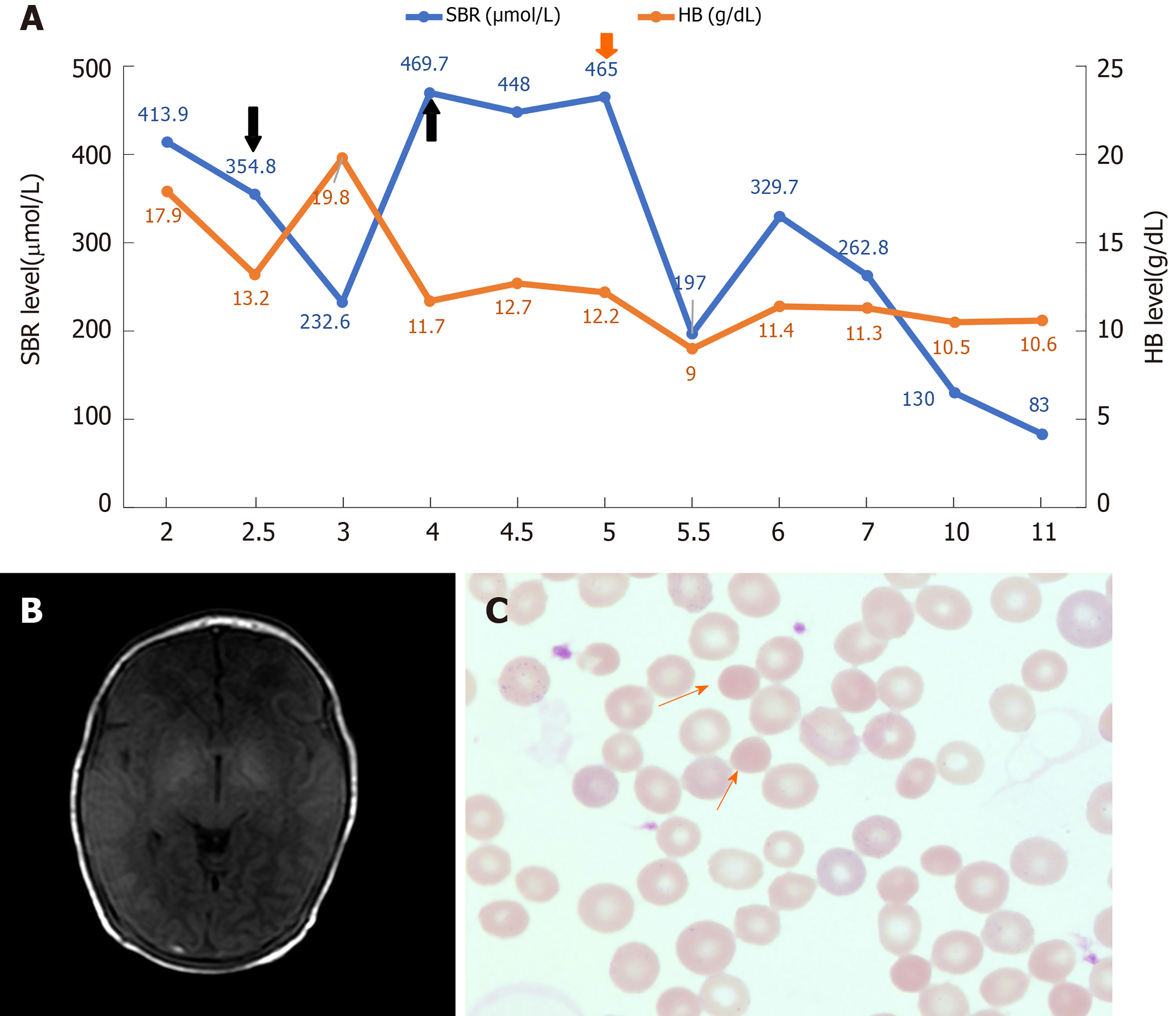
He was the first child of healthy, non-consanguineous Chinese parents without any known family history. He was born via cesarean delivery at a gestational age of 39+6 wk with APGAR scores of 9 at 1 and 5 min after birth. His birth weight was 3125 g (P10-P50), and his length was 48 cm (P10). He developed jaundice within 24 h after birth without apnea, fever, or seizures in the maternity hospital. Laboratory tests revealed serum bilirubin (SBR) of 413.9 µmol/L (direct 21.5) and Hb of 17.9 g/dL. The blood types of both the baby and the mother were B+. Double phototherapy and intravenous infusion of albumin were initiated. However, his bilirubin level did not significantly decrease (413.9 to 395.9 µmol/L) despite ongoing double phototherapy, and he was admitted to our neonatal intensive care unit 2 d after birth.
The patient had been hospitalized in a maternity hospital for 2 d.
He was the first child of healthy, non-consanguineous Chinese parents without any known family history.
Physical examination showed jaundice with neither pallor nor palpable liver and spleen. No other abnormalities on physical examination, such as poor sucking, decreased alertness/lethargy, high-pitched cry, hypertonia of the extensor muscles, and hypotonia, were detected.
The glucose-6-phosphate dehydrogenase deficiency screen and direct Coombs test were negative. Erythrocyte osmotic fragility was negative. Spherocytes were not observed on the blood smear. For the blood routine examination, the hemoglobin level was 13.2 g/dL (normal range, 15-23 g/dL), the mean corpuscular volume was 96.2 fL (normal range, 99-113 fL), the mean corpuscular hemoglobin was 34.9 pg (normal range, 27-32 pg), the mean corpuscular hemoglobin concentration was 36.2 g/dL (normal range, 31.7-33.0 g/dL), the standard deviation of red cell distribution width was 71.3 fL (37-54 fL), and the coefficient variation of red cell volume distribution width was 20.3% (11.0-16.0%). The SBR was 354.8 µmol/L (direct 37), with a reticulocyte of 12.18%.
Axial T1-weighted brain magnetic resonance imaging sequence revealed a bilateral, symmetrical, hyperintense signal in the globus pallidus without a mass effect, which suggested kernicterus (Figure 1B).
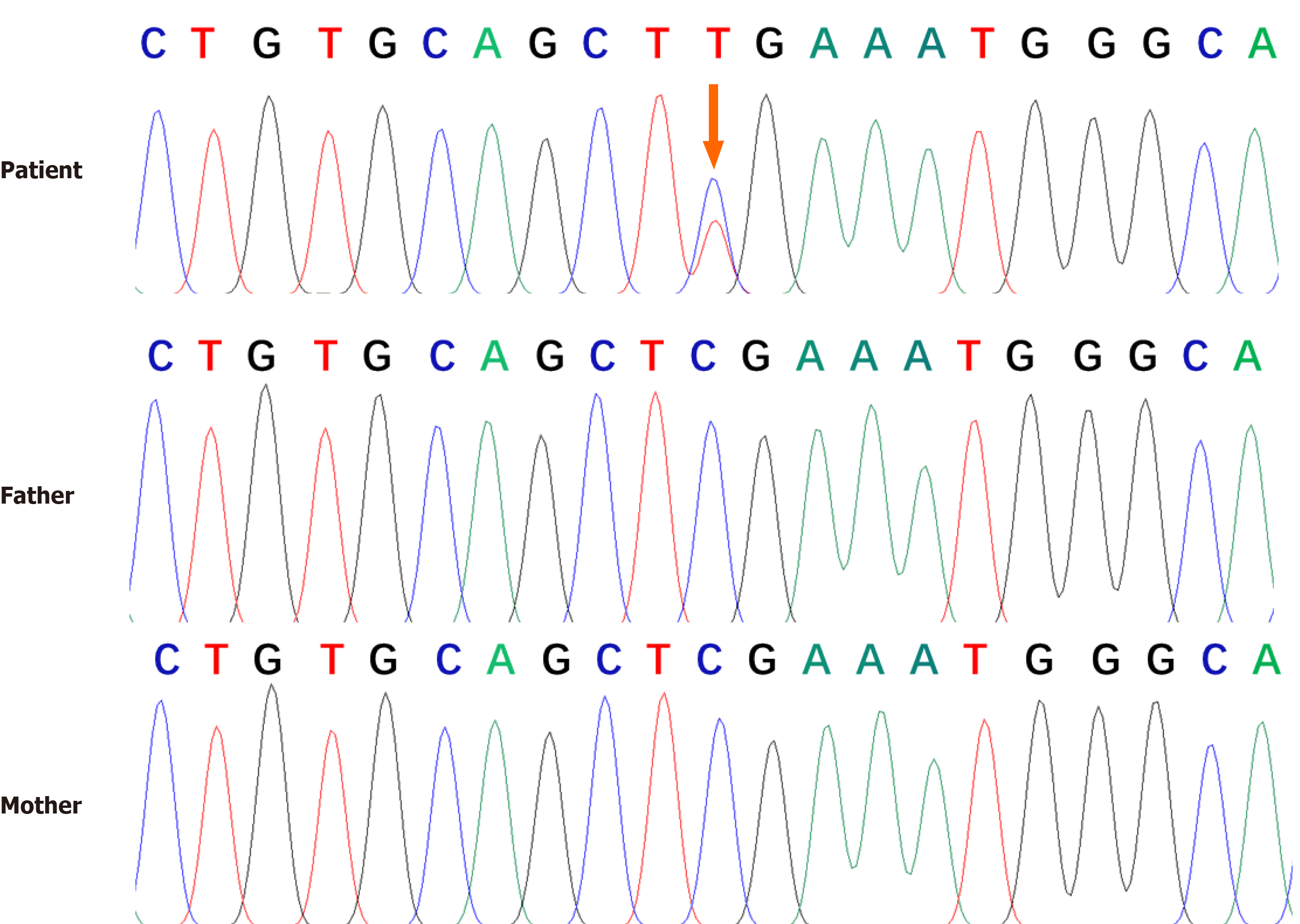
Genetic analysis revealed a heterozygous null mutation in the patient's ANK1 gene: c.841C > T(p.Arg281Ter). Sanger sequencing results showed that the heterozygous nonsense mutation was not detected in the patient's parents, indicating a de novo mutation (Figure 2). Combined with the above results, the patient was diagnosed with HS1.
Considering that this child had a rare but severe hyperbilirubinemia and neglected hemolytic disease, an exchange transfusion was performed via a continuous arterio-venous exchange. The SBR began falling, with subsequent measurements of 232.6 µmol/L. One day later, the SBR elevated to 469.7 µmol/L (direct 27.6), with Hb of 11.7 g/dL. The second exchange transfusion was performed, and the SBR decreased to 448 µmol/L. The next day SBR was elevated to 465 µmol/L. After two exchange transfusions without any obvious improvement, therapeutic plasma exchange was provided, which decreased serum bilirubin. The patient experienced a subsequent decrease in total bilirubin from 465 µmol/L to a nadir value of 197.0 µmol/L. No significant increase in bilirubin was observed under phototherapy.
After 4 d without phototherapy, bilirubin fluctuated between 83-130 µmol/L, and Hb was stable between 10.5-11.4 g/dL (Figure 1A). The patient did not have any abnormal neurologic manifestations and was discharged after 11 d of hospitalization. This patient has been followed up regularly. The spherocytes were observed on the blood smear at 6 mo of age (Figure 1C). To date, he has shown mild anemia and jaundice, but he has not experienced splenomegaly. No visual, auditory, or extrapyramidal abnor
In this report, we describe a Chinese male neonate with HS1, resulting in severe hyperbilirubinemia. A de novo mutation in exon 9: c.841C > T(p.Arg281Ter) caused the premature termination of peptide translation in exon 9 of ANK1, which was found through trio clinical exome sequencing followed by Sanger sequencing.
HS is an inherited membranopathy of red blood cells characterized by phenotypic and genotypic heterogeneity, making it difficult to diagnose during the first year of life[11]. During the perinatal period, the clinical manifestation of HS ranges from severe fetal anemia with hydrops fetalis to no clinical symptoms. HS is characterized by hemolytic anemia, jaundice, splenomegaly, cholelithiasis, and spherocytes, which are observed on the peripheral blood smear. The typical neonatal erythropoiesis response is slow, often making the reticulocyte count relatively low in comparison with anemia. Nevertheless, these symptoms are not typical in the newborn. More than one-half of neonates with HS are anemia-free in the first week of life. Rare cases exhibit splenomegaly, and spherocytes are observed in the blood smear of neonates[7]. Neonate jaundice is the most common and can be the only manifestation of neonatal HS. Our patient's serum total bilirubin was raised higher than 400 μmol/L within 24 h after birth and was prolonged, suggesting a high risk of kernicterus. Afterwards, his brain MRI results showed symmetrical hyperintensity of globus pallidus on T1-weighted sequence. Although the symmetric hyperintensity of globus pallidus on T1-weighted in neonates can be seen in "a transient hyperintensity" or other diseases including cerebral injury, hypoproteinemia, premature delivery, apnea, purulent meningitis, sepsis, etc., the patient did not appear to have any of these illnesses. In our case, he exhibited severe and delayed remission of jaundice and anemia without splenomegaly or spherocytes in the neonatal period. Therefore, we have reason to believe that patients with severe jaundice and anemia in the neonatal period should undergo genetic testing to confirm whether they have HS after excluding the possibility of other hemolytic diseases.
ANK1, located at 8p11.21[12], encodes erythroid ankyrin, and its mutations are the most common causes of HS1. ANK1 contains 43 exons with a complementary DNA length of 8300 bp. The erythroid ankyrin 1 protein consists of 1880 amino acids with three main structural domains, an N-terminal membrane-binding domain, a central spectrin binding domain, and a C-terminal regulatory domain[13]. All three domains of ANK1 are key to maintaining the shape of erythrocytes essential for proper function and assembly of the erythrocyte membranous-cytoskeletal network. Mutations in ANK1 can result in spherocytes with high osmotic fragility observed in most HS patients[14]. ANK1 mutations, mainly nonsense and frameshift mutations, can significantly affect the function of ANK1[15,16]. In this case, a de novo mutation at c.841C > T (p. Arg281Ter) in the ANK1 gene was identified as a nonsense mutation. This mutation led to premature termination of the protein at the spectrin binding domain, thereby forming a truncated protein without normal function, causing an early-onset and severe phenotype.
In the literature, we found a few cases diagnosed with HS1 during the neonatal period and compared their clinical features with our patient (Table 1). Case 2 was a male neonate who developed severe hemolytic anemia 4 wk after his birth and was identified to have a novel de novo nonsense E9X: c.25G > T(p.Glu9Ter) mutant in exon 1 of ANK1[17]. Case 3 was a 31-wk premature infant who received an RBC transfusion 3 d after birth because of severe anemia and jaundice; this patient was clinically diagnosed with HS1 and carried a de novo nonsense mutation (Q109X: c.325C > T(p.Gln109Ter) in exon 4 of ANK1[18]. In these cases, the early phenotype can be attributed to the severely impaired protein function by a nonsense mutation. It is worth highlighting a notable difference between our case and the other two cases, both of whom presented with severe anemia as the first manifestation. Our patient presented with intractable pathological neonatal jaundice resistant to phototherapy and exchange transfusion, explaining the different length of retained truncated proteins by other mutations. In short, HS1 caused by nonsense mutations in ANK1 tends to have an early onset and more severe phenotype. Although there are a few HS1 cases caused by other mutation types of ANK1 and have a certain degree of clinical phenotype in the neonatal and infancy period, the diagnosis time is often delayed[19,20]. In summary, the physicians should include HS1 in the differential diagnosis in patients who develop severe jaundice or anemia without any known reason in the neonatal period. Moreover, physicians should consider sequencing of relevant genes, especially for ANK1, which will provide beneficial help for the timely diagnosis of HS1.
| Patient | Case 1 | Case 2 | Case 3 | Reference |
| Gender | Male | Male | Male | |
| Gestational age | 39+6 wk | Term infant | 31 wk | |
| Appearance characteristics | ||||
| Time to first onset | Within 24 h after birth | 4 wk after birth | 3 d after birth | |
| Pallor | - | + | + | |
| Jaundice | Severe | Moderate | Moderate | |
| Laboratory examinations | ||||
| Hb (g/L) | 130 | 51 | 80 | 150-230 |
| MCV (fL) | 96.2 | 75.8 | 70.8 | 55.4-60.2 |
| MCHC (g/dL) | 36.2 | 36.0 | 33.0 | 31.7-33.0 |
| RET (%) | 12.2 | 13.4 | 8.2 | 0.5-1.5 |
| MCHC/MCV (%) | 37.6 | 47.4 | 46.6 | |
| Erythrocyte osmotic fragility | Negative | Positive | Positive | |
| Genetics | De novo heterozygous nonsense mutation in exon 9 of ANK1 (c.841C > T,p.Arg281Ter) | De novo heterozygous nonsense mutation in exon 1 of ANK1 (c.25G > T,p.Glu9Ter) | De novo heterozygous nonsense mutation in exon 4 of ANK1 (c.325C > T,p.Gln109Ter) | |
In conclusion, we report a Chinese neonate diagnosed as HS1 with severe hyperbilirubinemia caused by the heterozygous null mutation in the ANK1 gene: c.841C > T(p.Arg281Ter). It is worth noting that early genetic analysis plays an important role in the diagnosis of HS. Prompt treatment and anticipatory guidance are essential to prevent neonates' adverse outcomes with HS, so physicians should be more aware of signs of HS in the newborn period.
We are thankful for the members of the family for their participation and help in the study.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reddy R S-Editor: Wang JL L-Editor: Filipodia P-Editor: Xing YX
| 1. | Hao L, Li S, Ma D, Chen S, Zhang B, Xiao D, Zhang J, Jiang N, Jiang S, Ma J. Two novel ANK1 loss-of-function mutations in Chinese families with hereditary spherocytosis. J Cell Mol Med. 2019;23:4454-4463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Da Costa L, Suner L, Galimand J, Bonnel A, Pascreau T, Couque N, Fenneteau O, Mohandas N; Society of Hematology and Pediatric Immunology (SHIP) group; French Society of Hematology (SFH). Diagnostic tool for red blood cell membrane disorders: Assessment of a new generation ektacytometer. Blood Cells Mol Dis. 2016;56:9-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Wang C, Cui Y, Li Y, Liu X, Han J. A systematic review of hereditary spherocytosis reported in Chinese biomedical journals from 1978 to 2013 and estimation of the prevalence of the disease using a disease model. Intractable Rare Dis Res. 2015;4:76-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Eber SW, Gonzalez JM, Lux ML, Scarpa AL, Tse WT, Dornwell M, Herbers J, Kugler W, Ozcan R, Pekrun A, Gallagher PG, Schröter W, Forget BG, Lux SE. Ankyrin-1 mutations are a major cause of dominant and recessive hereditary spherocytosis. Nat Genet. 1996;13:214-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 141] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Delhommeau F, Cynober T, Schischmanoff PO, Rohrlich P, Delaunay J, Mohandas N, Tchernia G. Natural history of hereditary spherocytosis during the first year of life. Blood. 2000;95:393-397. [PubMed] [Cited in This Article: ] |
| 6. | Christensen RD, Henry E. Hereditary spherocytosis in neonates with hyperbilirubinemia. Pediatrics. 2010;125:120-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Christensen RD, Yaish HM, Gallagher PG. A pediatrician's practical guide to diagnosing and treating hereditary spherocytosis in neonates. Pediatrics. 2015;135:1107-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141:367-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Da Costa L, Galimand J, Fenneteau O, Mohandas N. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev. 2013;27:167-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 10. | Miraglia del Giudice E, Nobili B, Francese M, D'Urso L, Iolascon A, Eber S, Perrotta S. Clinical and molecular evaluation of non-dominant hereditary spherocytosis. Br J Haematol. 2001;112:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kar R, Rao S, Srinivas UM, Mishra P, Pati HP. Clinico-hematological profile of hereditary spherocytosis: experience from a tertiary care center in North India. Hematology. 2009;14:164-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lambert S, Yu H, Prchal JT, Lawler J, Ruff P, Speicher D, Cheung MC, Kan YW, Palek J. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990;87:1730-1734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 120] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Lux SE, John KM, Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990;344:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 430] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | King MJ, Zanella A. Hereditary red cell membrane disorders and laboratory diagnostic testing. Int J Lab Hematol. 2013;35:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Gallagher PG, Forget BG. Hematologically important mutations: spectrin and ankyrin variants in hereditary spherocytosis. Blood Cells Mol Dis. 1998;24:539-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007;21:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Gundel F, Eber S, Heep A. A new ankyrin mutation (ANK1 EXON E9X) causing severe hereditary spherocytosis in the neonatal period. Ann Hematol. 2011;90:231-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Liu S, Jiang H, Huang LY, Li DZ. A de novo ankyrin mutation (ANK1 Q109X) causing severe hereditary spherocytosis from preterm neonatal period. Ann Hematol. 2017;96:1067-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Huang TL, Sang BH, Lei QL, Song CY, Lin YB, Lv Y, Yang CH, Li N, Yang YH, Zhang XW, Tian X. A de novo ANK1 mutation associated to hereditary spherocytosis: a case report. BMC Pediatr. 2019;19:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Luo Y, Li Z, Huang L, Tian J, Xiong M, Yang Z. Spectrum of Ankyrin Mutations in Hereditary Spherocytosis: A Case Report and Review of the Literature. Acta Haematol. 2018;140:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |