Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.4998
Peer-review started: January 28, 2021
First decision: February 24, 2021
Revised: March 11, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: July 6, 2021
Pancreatic cancer (PC) is the seventh leading cause of cancer death worldwide. The vast majority of patients who have PC develop metastases, resulting in poor treatment effects. Although great progress in therapeutic approaches has been achieved in recent decades, extensive drug resistance still persists, representing a major hurdle to effective anticancer therapy for pancreatic ductal adenocarcinoma (PDAC). Therefore, there is an urgent need to better understand the drug resistance mechanisms and develop novel treatment strategies to improve patient outcomes. Numerous studies suggest that chemoresistance is closely related to epithelial-mesenchymal transition (EMT) of PDAC cells. Thus, this article summarizes the impact of EMT on PDAC from the perspective of chemotherapy resistance and discusses the possible novel applications of EMT inhibition to develop more effective drugs against PDAC.
Core Tip: This article reviews the role of epithelial-mesenchymal transition in the emergence of chemotherapy resistance in pancreatic ductal adenocarcinoma and summarizes the potential epithelial-mesenchymal transition targets to overcome chemoresistance.
- Citation: Hu X, Chen W. Role of epithelial-mesenchymal transition in chemoresistance in pancreatic ductal adenocarcinoma. World J Clin Cases 2021; 9(19): 4998-5006
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/4998.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.4998
Pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer (PC), is projected to be the second leading cause of cancerrelated death after lung cancer before 2030[1]. According to GLOBOCAN estimates, in 2018, there were 458918 new cases of PC, resulting in 432242 deaths[2]. PDAC is a complex and heterogeneous disease, involving a multitude of genetic, epigenetic, and other risk factors, such as smoking[3]. Currently, surgical resection followed by adjuvant chemotherapy remains the only potentially curative treatment for PDAC; however, only 20% patients are diagnosed early with locally resectable, non-metastatic disease[4,5]. Chemotherapy is available for the majority of patients who are diagnosed late with advanced disease and gives them hope. Despite the great progress made in the detection and treatment of PDAC, its prognosis remains dismal, with a five-year survival rate of approximately 9%[6]. These poor clinical outcomes are likely caused by the development of chemo
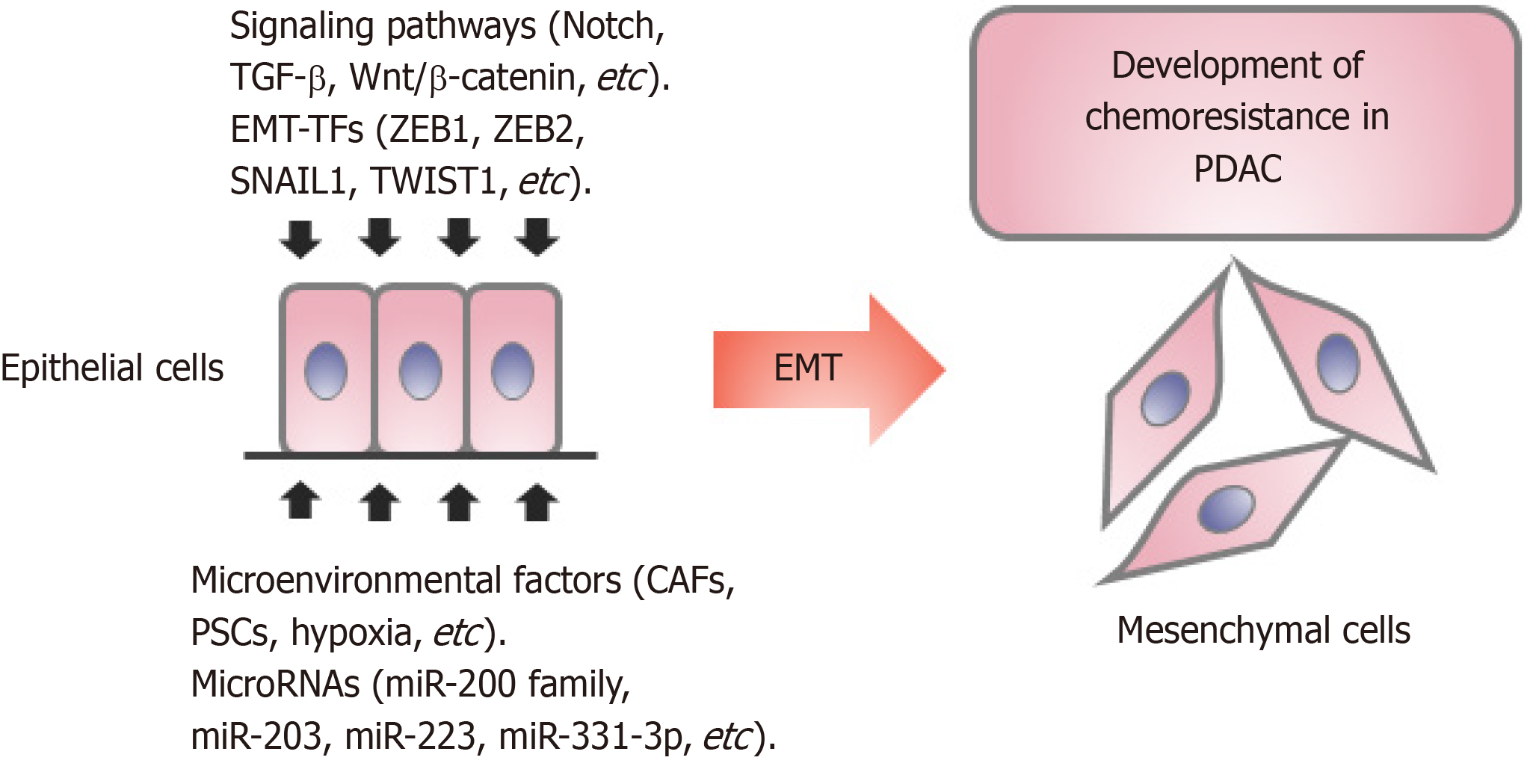
Chemoresistance, defined as cancer cells showing no or less response to drugs at the effective inhibitory concentration, is classified into primary and acquired resistance. Currently, folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin or nab-paclitaxel plus gemcitabine are considered first-line therapies, because they provide patients with a 4.3-mo and 1.8-mo increase, respectively, in median survival when compared with gemcitabine alone[7,8]. However, not all patients benefit from first-line therapy owing to chemoresistance. The mechanisms of drug resistance are complex, including the activities of drug transporters, the tumor microenvironment, epithelial-mesenchymal transition (EMT), and the effects of microRNAs (miRNAs), enzymes, and their targets[9]. The EMT process is a major contributor to the development of resistance in multiple cancer types[10]. To this end, we will mainly discuss the effect of EMT on PDAC chemoresistance.
Classical EMT involves a phenotypic change in cells, in which cells loss their epithelial phenotype, such as tight cell-to-cell adhesion and apical-basal polarity, and acquire a highly invasive, mesenchymal phenotype[11]. Molecularly, EMT results in downregulation of the epithelial marker Ecadherin while enhancing the expression of the mesenchymal factors (e.g., Ncadherin, fibronectin, SNAIL2, and vimentin)[12]. Initially, EMT was described as being essential for many stages in embryonic development, and was found subsequently to play a crucial role in adult tissue, such as organ fibrosis, wound healing, and metastasis[13,14]. In the past decades, extensive research has been conducted to investigate the role and regulation of EMT in tumor progression[15,16]. Accumulating evidence suggests that EMT plays an important role in the pathogenesis, invasion, metastasis, and drug resistance in PDAC[17-19].
In this review, we summarize the results of published studies on the role of EMT and the proposed EMT targets in drug-resistant PDAC, including a focus on the molecular mechanism of EMT in chemoresistance. Moreover, we also discuss EMT targeted therapy, and review the advantages and disadvantages of each approach.
EMT plays an important role in metastasis and is involved in several kinds of cancer, including PDAC[20]. Recently, studies have highlighted the importance of EMT in conferring chemoresistance in diverse cancers[17,21,22]. Although some studies showed that EMT makes a limited contribution to metastases, the role of EMT in conferring chemoresistance is clear in breast and pancreatic tumors[23,24].
Gemcitabine resistance is closely associated with EMT in PDAC. In 1996, gemcitabine was approved by the Food and Drug Administration to treat all stages of advanced PC, and it is still an important drug for the treatment of PC until now[25]. However, gemcitabine treatment provides limited survival benefit because of intrinsic or acquired resistance[26]. PDAC cell lines (BxPC3 and PANC-1) have different intrinsic gemcitabine resistance profiles: BxPC3 cells with an epithelial-like phenotype are more chemosensitive to gemcitabine than PANC1 cells with a mesenchymal-like phenotype[27]. El Amrani et al[28] reported that gemcitabine treatment induces EMT-like changes that are mediated by the extracellular regulated kinase (ERK)-zinc finger E-box binding homeobox 1 (ZEB1) pathway, and inhibition of ERK1/2 phospho
EMT causes resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in PC (Figure 1). Erlotinib (a first-generation EGFR-TKI) in combination with gemcitabine has been approved to treat PC in the USA and Europe[37]. Pancreatic cell lines that have higher expression of ZEB1, SNAIL1, and TWIST and have undergone EMT show a reduced sensitivity to erlotinib[38]. Epithelial tumor cells are significantly more sensitive to EGFR inhibitors than tumor cells with mesenchymal-like characteristics in pancreatic carcinomas[37]. In addition, a study demonstrated that the TGFβ-miR200-MIG6 pathway orchestrates the EMT-associated kinase switch that induces resistance to EGFR inhibitors[39]. Brexpiprazole reverses osimertinib (a third-generation EGFR-TKI) resistance in lung cancer and PC by suppressing survivin, which could activate transforming growth factor-β (TGF-β)/SMAD signaling, thus causing EMT[40,41].
EMT is involved in resistance to other chemotherapeutic agents in PC. According to Arumugam et al[17], ZEB1 regulates E-cadherin expression and the sensitivity to 5-fluorouracil (5-FU) and cisplatin treatment negatively, and ZEB1 silencing upregulated epithelial markers (E-cadherin, EVA1, and MAL2) and restored 5-FU and cisplatin sensitivity in several drug-resistant cell lines (PANC-1, MIAPaCa-2, and Hs766T). EMT inhibition sensitized PDAC cells to 5-FU, and CHL1 overexpression rescued 5-FU chemoresistance via the Hedgehog (Hh) pathway[42]. Mocetinostat, a histone deacetylase (HDAC) inhibitor, inhibited ZEB1 by restoring miR-203 expression, reversing the EMT process in GR PC cells, and sensitizing the cells to docetaxel[43].
EMT is induced by several pathways, mainly including the TGF-β, Notch, Wnt/β catenin, Hh, tumor necrosis factor-α (TNF-α), HIF-1α, nuclear factor kappa B (NF-κB), and receptor tyrosine kinase signaling pathways[44]. Notch receptor-1 (Notch-1) is overexpressed in GR PC cells and plays an important role in GR-induced EMT[45]. Notch-2 activation was shown to mediate a chemoresistant phenotype (EMT phenotype) in GR PDAC cells, and downregulation of Notch signaling reversed the EMT phenotype partially to induce mesenchymal-epithelial transition (MET)[46]. Furthermore, Gungor et al[47] reported that gemcitabine induced Midkine (MK), a heparin-binding growth factor that is widely overexpressed in several types of cancers, the depletion of which was linked to increased sensitivity to gemcitabine treatment. Taken together, Notch signaling is activated by MK-derived EMT, which upregulates NFκB and increases chemoresistance in PDAC.
The expression pattern of hMENA isoforms, which was regulated by TGF-β1, played a crucial role in TGF-β1-induced EMT, and might represent promising targets to develop new prognostic and therapeutic tools in PDAC[48]. Zhan et al[49] found that in gemcitabine-treated PC cells, miR-331-3p was upregulated, which activated Wnt/β-catenin signaling via ST7L, while miR-331-3p inhibition and ST7L overexpression restored the activation of Wnt/β-catenin signaling and decreased drug resistance.
EMT is regulated at the cellular level by certain zinc finger transcription factors, mainly of the SNAIL, TWIST, and ZEB families[13,50]. These EMT-activating transcription factors (EMT-TFs) play pleiotropic roles in tumor progression and have been associated with poor clinical outcome in human cancers. Although the EMT process is reactivated in cancers, the end-stage markers, such as vimentin, are usually not expressed[13]. In addition, cancer cells often undergo partial EMT, and both epithelial and mesenchymal markers are expressed in the same cell. Therefore, attention must be paid to EMT-TFs, and not just to the prototypical EMT markers, such as E-cadherin and vimentin. As inhibitors of the epithelial phenotype, ZEB1, ZEB2, SNAIL1, SNAIL2, and TWIST1 are not expressed in normal epithelial cells, but are highly expressed in invading dedifferentiated cancer cells of pancreatic carcinomas[50,51]. Silencing of EMTTFs (SNAIL1, SNAIL2, and TWIST) expression using short hairpin RNA or small molecule inhibitors of EMT, such as CX4945 and SD208, reduced EMT metastasis, stem cell properties, and drug resistance (5-FU and Mitomycin C) of PC cell lines[52]. Namba et al[53] reported that the AKT-GSK3β-SNAIL pathway was inhibited using Zidovudine, an anti-viral drug, which could reverse EMT and overcome gemcitabine resistance of PC cells.
Wellner et al[54] reported that ZEB1 not only activated EMT via a stemnessinhibiting miRNA, but was also necessary for the tumorinitiating capacity of PC cells, and targeting the ZEB1-miR-200 feedback loop might be a promising treatment for PC. This finding suggested that in addition to directly targeting EF-TFs, miRNAs are also a good target for indirect inhibition of EMT-TFs.
MiRNAs are a class of small non-coding RNAs shorter than 22 nucleotides, which play a crucial role in the progression and chemoresistance of PDAC[55]. For example, Song et al[56] reported that miRNA-21 was overexpressed in patients with GR PDAC compared with that in patients with gemcitabine-sensitive PDAC, and inhibition of miRNA-21 could reverse invasion and metastasis via the PTEN/AKT pathway. Moreover, Liu et al[57] found that miR-125a-3p was downregulated in a time-dependent manner after treatment with gemcitabine, and upregulation of miR-125a-3p increased chemosensitivity to gemcitabine significantly and inhibited the EMT by targeting FYN in PDAC cells. A number of miRNAs that regulate EMT and PDAC drug resistance have been identified, and some of them are summarized in Table 1. It is clear that miRNAs could be promising targets to inhibit EMT to overcome chemoresistance in PDAC.
| miRNA | Signaling axis | Function | Ref. |
| miR-200 | MiR-200/ZEB1/EMT | MiR-200 inhibited EMT and increased the sensitivity of GR PC cells to gemcitabine | [34] |
| miR-141 | MiR-141/TM4SF1/AKT/EMT | MiR-141 inhibited EMT and reduced TM4SF1 expression by suppressing AKT signaling pathway | [35] |
| miR-203 | MiR203-ZEB1-EMT | MiR-203 inhibited EMT and increased the sensitivity to gemcitabine | [43] |
| miR-223 | MiR-223/Fbw7/Notch-1/EMT | MiR-223 induced EMT and conferred gemcitabine-resistance by downregulation of Fbw7 and subsequent upregulation of Notch-1 | [45] |
| miR-331-3p | miR-331-3p/ST7L/Wnt/β-catenin/EMT | MiR-331-3p induced EMT and conferred gemcitabine-resistance by activating the Wnt/β-catenin signaling pathway via ST7L | [49] |
| miR-21 | miR-21/PTEN/Akt | MiR-21 induced invasion, and metastasis, and conferred gemcitabine-resistance by miR-21/PTEN/Akt | [56] |
| miR-125a-3p | miR-125a-3p/Fyn/EMT | MiR-125a-3p inhibited EMT and increased chemosensitivity to gemcitabine by directly targeting Fyn | [57] |
| miR-145 | miR-145/ ZEB1/EMT | MiR-145 inhibited EMT and reversed acquired gemcitabine resistance | [62] |
Given the pivotal role that EMT plays in tumor progression, EMT is considered a target for cancer therapy. Although there are many problems that remain to be resolved, marked progress has been made in the development of anti-EMT agents to overcome chemoresistance in cancer. Recently, several screening strategies have been proposed to identify EMT inhibitors, which were summarized by Marcucci et al[58]. Screening strategies to inhibit the EMT pathway to overcome chemoresistance mainly include inhibiting EMT induction, promoting MET, and targeting mesenchymal tumor cells. Inhibitors of EMT induction might be effective to prevent chemoresistance, and cancer cells that have already undergone EMT might benefit from compounds promoting MET[59].
It is clear that targeting a single receptor, enzyme, or transporter protein involved in EMT has limitations, because EMT is not a uniform process defined by a single pathway. Targeting EMT-TFs or miRNAs with pleiotropic function might be an effective approach to inhibit metastasis while overcoming chemoresistance. In addition, EMT-TFs and miRNAs can form a feedback loop to regulate each other, depending on environmental triggers. For example, ZEB1 directly suppresses the expression of miR-200 family members (miR-141 and miR-200c) and ZEB1 is the predominant target downregulated by these miRNAs. Triggering the ZEB1-miR-200 feedback loop promotes EMT and invasion in PDAC[54,60]. However, there is still a long way to go to achieve targeting of EMT-TFs and miRNAs because of inefficient intracellular delivery in vivo. As an alternative approach, small molecule inhibitors (such as Mocetinostat, as mentioned above) are waiting to be discovered.
The composition of the tumor microenvironment is also an attractive target because it makes an important contribution to EMT-driven drug resistance in PDAC[9,21]. Inflammation is an important factor in the tumor microenvironment and contributes to the chemoresistance of PDAC cells indirectly via EMT induction, resulting in poor survival rates[61]. In addition, inhibitors of HIF-1α, a hypoxia-induced transcription factor, might be promising drugs to inhibit chemoresistance stimuli[58].
In summary, resistance to several chemotherapies, including gemcitabine, erlotinib, 5-FU, and cisplatin, in PDAC is mediated by EMT. Therefore, the EMT pathway has great therapeutic significance to overcome chemoresistance in PDAC. EMT is regulated by several pathways, such as TGF-β, Notch, and Wnt/β catenin signaling pathways. Although many studies have explored the role of EMT in chemotherapy-resistant PDAC, the mechanism is unclear and further studies are required. The EMT process is executed via EMT-TFs; therefore, it can be inhibited by targeting EMT-TFs in its initial stage. In addition, targeting EMT-TFs and miRNAs, and inhibiting stimuli of chemoresistance might be effective to ameliorate EMT-driven drug resistance in PDAC. Despite certain limitations, we can be optimistic about the efficacy of anti-EMT compounds, which might overcome chemoresistance of PDAC cells in the near future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carloni R S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3888] [Cited by in F6Publishing: 4589] [Article Influence: 458.9] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 50871] [Article Influence: 8478.5] [Reference Citation Analysis (44)] |
| 3. | Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 466] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 4. | Khomiak A, Brunner M, Kordes M, Lindblad S, Miksch RC, Öhlund D, Regel I. Recent Discoveries of Diagnostic, Prognostic and Predictive Biomarkers for Pancreatic Cancer. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Löhr M. Is it possible to survive pancreatic cancer? Nat Clin Pract Gastroenterol Hepatol. 2006;3:236-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 1213] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 7. | Aroldi F, Bertocchi P, Savelli G, Rosso E, Zaniboni A. Pancreatic cancer: New hopes after first line treatment. World J Gastrointest Oncol. 2016;8:682-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Passero FC Jr, Saif MW. Second line treatment options for pancreatic cancer. Expert Opin Pharmacother. 2017;18:1607-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in Pancreatic Cancer. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 10. | Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1241] [Cited by in F6Publishing: 1630] [Article Influence: 232.9] [Reference Citation Analysis (0)] |
| 11. | Rodriguez-Aznar E, Wiesmüller L, Sainz B Jr, Hermann PC. EMT and Stemness-Key Players in Pancreatic Cancer Stem Cells. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Krantz SB, Shields MA, Dangi-Garimella S, Bentrem DJ, Munshi HG. Contribution of epithelial-mesenchymal transition to pancreatic cancer progression. Cancers (Basel). 2010;2:2084-2097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1104] [Cited by in F6Publishing: 1261] [Article Influence: 210.2] [Reference Citation Analysis (0)] |
| 14. | Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel). 1995;154:8-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1088] [Cited by in F6Publishing: 1106] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 15. | Recondo G, Mezquita L, Facchinetti F, Planchard D, Gazzah A, Bigot L, Rizvi AZ, Frias RL, Thiery JP, Scoazec JY, Sourisseau T, Howarth K, Deas O, Samofalova D, Galissant J, Tesson P, Braye F, Naltet C, Lavaud P, Mahjoubi L, Abou Lovergne A, Vassal G, Bahleda R, Hollebecque A, Nicotra C, Ngo-Camus M, Michiels S, Lacroix L, Richon C, Auger N, De Baere T, Tselikas L, Solary E, Angevin E, Eggermont AM, Andre F, Massard C, Olaussen KA, Soria JC, Besse B, Friboulet L. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in ALK-Rearranged Lung Cancer. Clin Cancer Res. 2020;26:242-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 16. | Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis AK, Huang RJY, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massagué J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G; EMT International Association (TEMTIA). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 624] [Cited by in F6Publishing: 1006] [Article Influence: 251.5] [Reference Citation Analysis (0)] |
| 17. | Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820-5828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 685] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 18. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1400] [Cited by in F6Publishing: 1521] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 19. | Alvarez MA, Freitas JP, Mazher Hussain S, Glazer ES. TGF-β Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J Gastrointest Cancer. 2019;50:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 783] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 21. | Du B, Shim JS. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules. 2016;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 494] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 22. | Huang J, Li H, Ren G. Epithelial-mesenchymal transition and drug resistance in breast cancer (Review). Int J Oncol. 2015;47:840-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1196] [Cited by in F6Publishing: 1341] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 24. | Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1539] [Article Influence: 171.0] [Reference Citation Analysis (0)] |
| 25. | Barton-Burke M. Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs. 1999;22:176-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Cao H, LE D, Yang LX. Current status in chemotherapy for advanced pancreatic adenocarcinoma. Anticancer Res. 2013;33:1785-1791. [PubMed] [Cited in This Article: ] |
| 27. | Kim Y, Han D, Min H, Jin J, Yi EC, Kim Y. Comparative proteomic profiling of pancreatic ductal adenocarcinoma cell lines. Mol Cells. 2014;37:888-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | El Amrani M, Corfiotti F, Corvaisier M, Vasseur R, Fulbert M, Skrzypczyk C, Deshorgues AC, Gnemmi V, Tulasne D, Lahdaoui F, Vincent A, Pruvot FR, Van Seuningen I, Huet G, Truant S. Gemcitabine-induced epithelial-mesenchymal transition-like changes sustain chemoresistance of pancreatic cancer cells of mesenchymal-like phenotype. Mol Carcinog. 2019;58:1985-1997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Tsukasa K, Ding Q, Yoshimitsu M, Miyazaki Y, Matsubara S, Takao S. Slug contributes to gemcitabine resistance through epithelial-mesenchymal transition in CD133(+) pancreatic cancer cells. Hum Cell. 2015;28:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Barati Bagherabad M, Afzaljavan F, ShahidSales S, Hassanian SM, Avan A. Targeted therapies in pancreatic cancer: Promises and failures. J Cell Biochem. 2019;120:2726-2741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Zhao H, Duan Q, Zhang Z, Li H, Wu H, Shen Q, Wang C, Yin T. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med. 2017;21:2055-2067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Wang X, Song Z, Chen F, Yang X, Wu B, Xie S, Zheng X, Cai Y, Chen W, Zhong Z. AMPK-related kinase 5 (ARK5) enhances gemcitabine resistance in pancreatic carcinoma by inducing epithelial-mesenchymal transition. Am J Transl Res. 2018;10:4095-4106. [PubMed] [Cited in This Article: ] |
| 33. | Wang S, Li MY, Liu Y, Vlantis AC, Chan JY, Xue L, Hu BG, Yang S, Chen MX, Zhou S, Guo W, Zeng X, Qiu S, van Hasselt CA, Tong MC, Chen GG. The role of microRNA in cisplatin resistance or sensitivity. Expert Opin Ther Targets. 2020;24:885-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Li Y, VandenBoom TG 2nd, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704-6712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 538] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 35. | Xu D, Yang F, Wu K, Xu X, Zeng K, An Y, Xu F, Xun J, Lv X, Zhang X, Yang X, Xu L. Lost miR-141 and upregulated TM4SF1 expressions associate with poor prognosis of pancreatic cancer: regulation of EMT and angiogenesis by miR-141 and TM4SF1 via AKT. Cancer Biol Ther. 2020;21:354-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Zhao T, Jin F, Xiao D, Wang H, Huang C, Wang X, Gao S, Liu J, Yang S, Hao J. IL-37/ STAT3/ HIF-1α negative feedback signaling drives gemcitabine resistance in pancreatic cancer. Theranostics. 2020;10:4088-4100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Barr S, Thomson S, Buck E, Russo S, Petti F, Sujka-Kwok I, Eyzaguirre A, Rosenfeld-Franklin M, Gibson NW, Miglarese M, Epstein D, Iwata KK, Haley JD. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis. 2008;25:685-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, Iwata KK, Gibson NW, Cagnoni P, Haley JD. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 39. | Izumchenko E, Chang X, Michailidi C, Kagohara L, Ravi R, Paz K, Brait M, Hoque MO, Ling S, Bedi A, Sidransky D. The TGFβ-miR200-MIG6 pathway orchestrates the EMT-associated kinase switch that induces resistance to EGFR inhibitors. Cancer Res. 2014;74:3995-4005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Sanomachi T, Suzuki S, Togashi K, Seino S, Yoshioka T, Kitanaka C, Okada M, Yamamoto M. Brexpiprazole Reduces Survivin and Reverses EGFR Tyrosine Kinase Inhibitor Resistance in Lung and Pancreatic Cancer. Anticancer Res. 2019;39:4817-4828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Zhao X, Yang Y, Yu H, Wu W, Sun Y, Pan Y, Kong L. Polydatin inhibits ZEB1-invoked epithelial-mesenchymal transition in fructose-induced liver fibrosis. J Cell Mol Med. 2020;24:13208-13222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Li H, Jiang W, Liu XN, Yuan LY, Li TJ, Li S, Xu SS, Zhang WH, Gao HL, Han X, Wang WQ, Wu CT, Yu XJ, Xu HX, Liu L. TET1 downregulates epithelial-mesenchymal transition and chemoresistance in PDAC by demethylating CHL1 to inhibit the Hedgehog signaling pathway. Oncogene. 2020;39:5825-5838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Meidhof S, Brabletz S, Lehmann W, Preca BT, Mock K, Ruh M, Schüler J, Berthold M, Weber A, Burk U, Lübbert M, Puhr M, Culig Z, Wellner U, Keck T, Bronsert P, Küsters S, Hopt UT, Stemmler MP, Brabletz T. ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol Med. 2015;7:831-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 44. | Garg M. Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer. World J Stem Cells. 2013;5:188-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 45. | Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, Wang Z. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740-1749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 465] [Cited by in F6Publishing: 516] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 47. | Güngör C, Zander H, Effenberger KE, Vashist YK, Kalinina T, Izbicki JR, Yekebas E, Bockhorn M. Notch signaling activated by replication stress-induced expression of midkine drives epithelial-mesenchymal transition and chemoresistance in pancreatic cancer. Cancer Res. 2011;71:5009-5019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Melchionna R, Iapicca P, Di Modugno F, Trono P, Sperduti I, Fassan M, Cataldo I, Rusev BC, Lawlor RT, Diodoro MG, Milella M, Grazi GL, Bissell MJ, Scarpa A, Nisticò P. The pattern of hMENA isoforms is regulated by TGF-β1 in pancreatic cancer and may predict patient outcome. Oncoimmunology. 2016;5:e1221556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Zhan T, Chen X, Tian X, Han Z, Liu M, Zou Y, Huang S, Chen A, Cheng X, Deng J, Tan J, Huang X. MiR-331-3p Links to Drug Resistance of Pancreatic Cancer Cells by Activating WNT/β-Catenin Signal via ST7L. Technol Cancer Res Treat. 2020;19:1533033820945801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Sánchez-Tilló E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429-3456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 51. | Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769-4776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 327] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 52. | Kaşıkcı E, Aydemir E, Bayrak ÖF, Şahin F. Inhibition of Migration, Invasion and Drug Resistance of Pancreatic Adenocarcinoma Cells - Role of Snail, Slug and Twist and Small Molecule Inhibitors. Onco Targets Ther. 2020;13:5763-5777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Namba T, Kodama R, Moritomo S, Hoshino T, Mizushima T. Zidovudine, an anti-viral drug, resensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine by inhibition of the Akt-GSK3β-Snail pathway. Cell Death Dis. 2015;6:e1795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1262] [Cited by in F6Publishing: 1339] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 55. | Duguang L, Jin H, Xiaowei Q, Peng X, Xiaodong W, Zhennan L, Jianjun Q, Jie Y. The involvement of lncRNAs in the development and progression of pancreatic cancer. Cancer Biol Ther. 2017;18:927-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 56. | Song WF, Wang L, Huang WY, Cai X, Cui JJ, Wang LW. MiR-21 upregulation induced by promoter zone histone acetylation is associated with chemoresistance to gemcitabine and enhanced malignancy of pancreatic cancer cells. Asian Pac J Cancer Prev. 2013;14:7529-7536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Liu G, Ji L, Ke M, Ou Z, Tang N, Li Y. miR-125a-3p is responsible for chemosensitivity in PDAC by inhibiting epithelial-mesenchymal transition via Fyn. Biomed Pharmacother. 2018;106:523-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016;15:311-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 59. | Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci. 2014;35:479-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 60. | Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1351] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 61. | Khalafalla FG, Khan MW. Inflammation and Epithelial-Mesenchymal Transition in Pancreatic Ductal Adenocarcinoma: Fighting Against Multiple Opponents. Cancer Growth Metastasis. 2017;10:1179064417709287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Gao Y, Zhang Z, Li K, Gong L, Yang Q, Huang X, Hong C, Ding M, Yang H. Linc-DYNC2H1-4 promotes EMT and CSC phenotypes by acting as a sponge of miR-145 in pancreatic cancer cells. Cell Death Dis. 2017;8:e2924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |