Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2466
Revised: March 17, 2024
Accepted: April 12, 2024
Published online: May 26, 2024
Fluorine-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography/com
Core Tip: The discovery of incidental focal colorectal fluorine-18 fluorodeoxyglucose uptake on positron emission tomography/computed tomography is not uncommon in clinical settings. This phenomenon presents a unique opportunity to delve into its nuanced implications and clinical relevance. In the forthcoming discourse, we aim to explore the intricate details of these unexpected findings, shedding light on the diagnostic challenges they pose and their potential impact on patient outcomes.
- Citation: Lee H, Hwang KH. Focal incidental colorectal fluorodeoxyglucose uptake: Should it be spotlighted? World J Clin Cases 2024; 12(15): 2466-2474
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2466.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2466
Since the introduction of fluorine-18 fluorodeoxyglucose (F-18 FDG) for clinical imaging, it has emerged as a pivotal oncologic imaging radiopharmaceutical in the domain of nuclear medicine. F-18 FDG serves as a prominent radioactive tracer for utilization in positron emission tomography (PET) or PET/computed tomography (PET/CT). While PET alone provides functional images with limited anatomical detail, the integration of simultaneous anatomical imaging within the same machine in hybrid PET/CT enables precise localization of FDG metabolism. Given that biochemical alterations precede physical manifestations, the molecular-level insights offered by PET/CT play a crucial role in the early detection of disease states[1,2].
The initial impediment to widespread F-18 FDG use was the necessity for a cyclotron and a radiochemical laboratory for production. However, advancements over time have led to the deployment of this equipment in various regions, sig
In contrast to other imaging modalities like CT or magnetic resonance imaging, the F-18 FDG PET/CT scan encompasses a broad range, spanning from the skull base to the upper thigh (torso), or in some instances, the entire body. This extensive scan range may encompass areas of lesser interest, potentially leading to the observation of increased FDG metabolism (hypermetabolism) at unexpected sites. This article delves into the distinctive characteristics of FDG, presenting imaging findings and exploring the clinical significance of incidental focal colorectal hypermetabolism.
The initial synthesis of FDG, credited to Pacák et al[3] in 1968, marked a significant milestone in medical imaging. F-18 FDG, developed by Ido et al in 1978, revolutionized PET imaging and found widespread application in oncology, neurology, and cardiology[3-6]. Structurally based on glucose, FDG substitutes the hydroxyl group on the 2-carbon of a glucose molecule with the fluorine-18 radionuclide[7,8]. Cellular uptake of this glucose analogue occurs primarily through glucose transporters 1 and 3, mirroring the uptake of natural glucose molecules into cells[7-9]. However, due to structural differences, FDG cannot complete the glucose metabolic pathway and becomes trapped within cells[10]. Despite this, the common initial metabolic behavior between FDG and glucose allows FDG to effectively evaluate and represent glucose metabolism in cells.
Living cells need glucose as energy source. The phenomenon known as the Warburg effect elucidates the heightened glucose uptake by cancer cells compared to normal cells for energy production[11]. Cancer cells prefer glycolysis over oxidative phosphorylation for energy production, despite its lower efficiency, as the rapid process aligns with the energy demands of cancer cells[12-15]. The increased glycolytic rate facilitates FDG uptake in cancer cells, enabling visualization through PET[16]. However, it is crucial to note that FDG is not exclusive to cancer cells. Organs with naturally high glucose metabolism, such as the brain or liver, exhibit increased FDG uptake. Moreover, benign conditions with elevated glycolysis also accumulate FDG in cells[17-21]. In essence, FDG demonstrates no discriminatory ability between malig
The assessment of accumulated FDG involves both visual interpretation and quantitative measurement. The semi-quantitative index known as the standardized uptake value (SUV) serves as a representative dimensionless ratio indi
The widespread application of SUV is evident in its utility for distinguishing malignant from benign lesions. This is particularly crucial in oncological diagnostics, where determining a cutoff specific to a particular cancer facilitates reference value comparisons. Moreover, SUV plays a pivotal role in the assessment of treatment efficacy, enabling the comparison of pre- and post-therapy imaging data. Various metrics can express SUV, including the maximum SUV (SUVmax) representing the highest value in a single pixel, the mean SUV (SUVmean) derived from the average value in a freely drawn region, and the peak SUV (SUVpeak) determined as the average SUV in a small fixed-sized region centered on a high uptake portion. While SUVmax maintains consistency across different measurements, it is susceptible to noise[23,24]. SUVmean, on the other hand, is sensitive to variations induced by the delineated area[25,26]. SUVpeak, encompassing a relatively large volume, exhibits resilience against noise interference but may pose challenges in the analysis of small or tiny lesions[27-29]. Additionally, alternative SUV-related parameters such as SUV corrected for lean body mass, metabolic tumor volume, and total lesion glycolysis find application in diverse clinical scenarios. Regrettably, no singular parameter emerges as flawless in addressing all aspects of SUV assessment.
The degree of FDG uptake is subject to variation, influenced by factors such as cellularity, cell activity, tumor size, and the local microenvironment[30-33]. It is important to recognize that not all cancer cells experience glucose deprivation, and FDG accumulation may not always be pronounced within them. In colorectal cancer, SUVmax values exhibited vari
Given the shared plasma membrane protein transport for both glucose and FDG, blood glucose concentration plays a role in FDG transport. Early studies in the 1990s highlighted that elevated blood sugar levels adversely affected image quality, as FDG competed with blood glucose for cellular membrane transport[39-42]. Current guidelines from the European Association of Nuclear Medicine and the Society of Nuclear Medicine and Molecular Imaging recommend conducting F-18 FDG PET/CT when blood glucose is controlled, ideally below 11 mmol/L (approximately 200 mg/dL)[16,43]. While recent literature suggests limited impact of blood glucose levels on imaging outcomes[44-48], adherence to published guidelines remains widespread.
The heightened glycolytic activity of cancer cells contributes to increased FDG uptake, resulting in prominently intense PET imaging[49-51]. SUV is a widely used metric in clinical settings, although its utility is not without limitations. An SUV of 2.5 or higher generally indicates potential malignancy, with an SUVmax of 3.5 to 4 suggested for colon lesions[52]. However, it is crucial to note that both malignant and non-malignant cells can exhibit elevated SUV due to the non-specific uptake mechanism of FDG[53-57]. It is well known that active infectious/inflammatory lesions or benign polyps may present high FDG uptake mimicking malignant lesions[58-62]. Additionally, physiological gastrointestinal FDG uptake is also common and intense colonic FDG uptake with metformin is well known[63-67]. Thus, interpreting high FDG uptake alone does not provide a definitive distinction between malignant and non-malignant lesions. FDG remains impartial in its diagnostic specificity.
When the criteria for heightened FDG uptake are satisfied, resulting in a conspicuous elevation in FDG metabolism within the image, the manifestation of uptake can assume a focal and/or diffuse nature. While not universally applicable, diffuse uptake in certain organs is more likely to be benign or physiological in origin than malignant[54,56,68-71]. In contrast, focal uptake holds greater clinical significance, necessitating careful consideration to avoid overlooking the potential presence of a malignant lesion[72-75]. The uptake pattern is of importance.
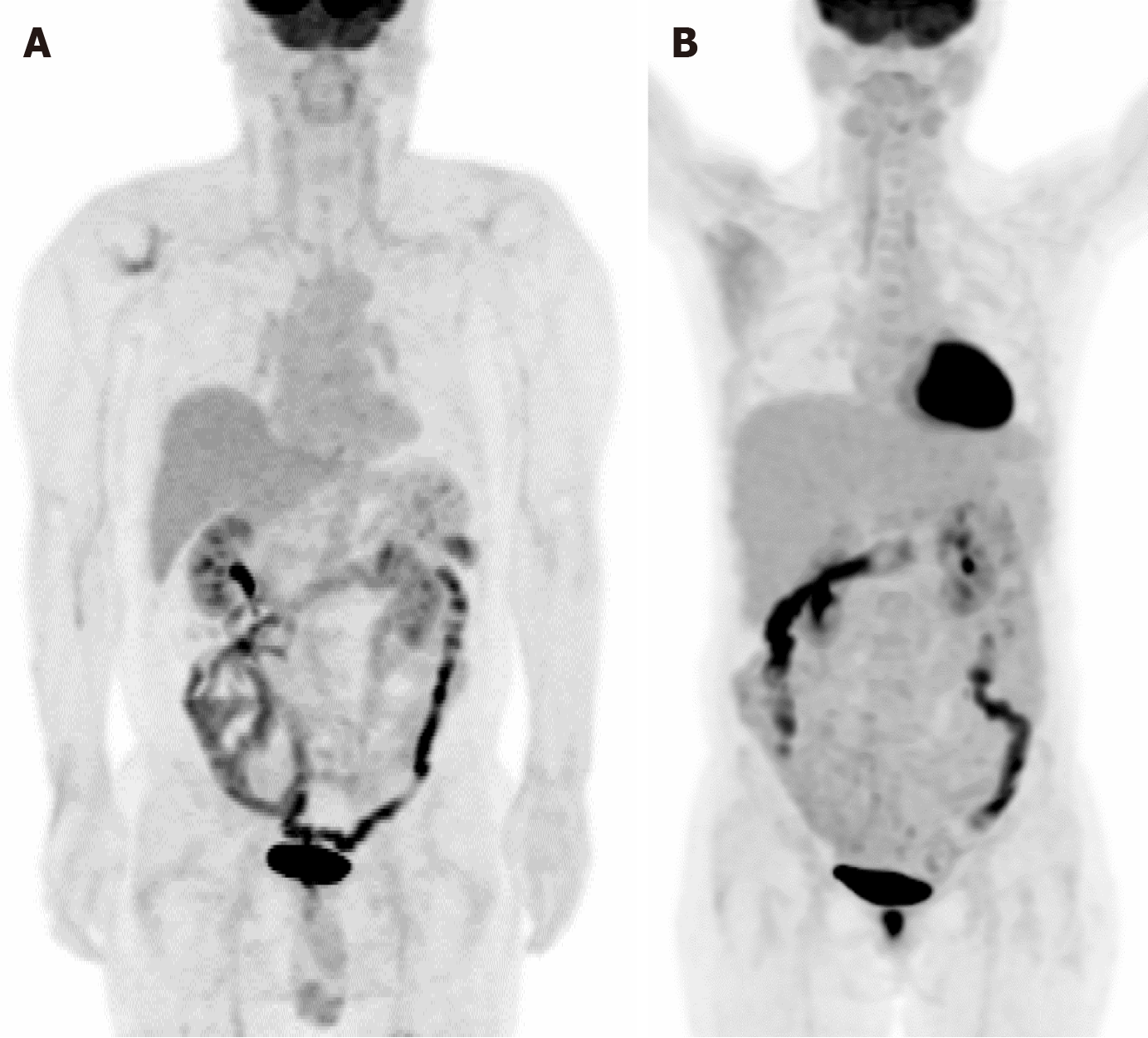
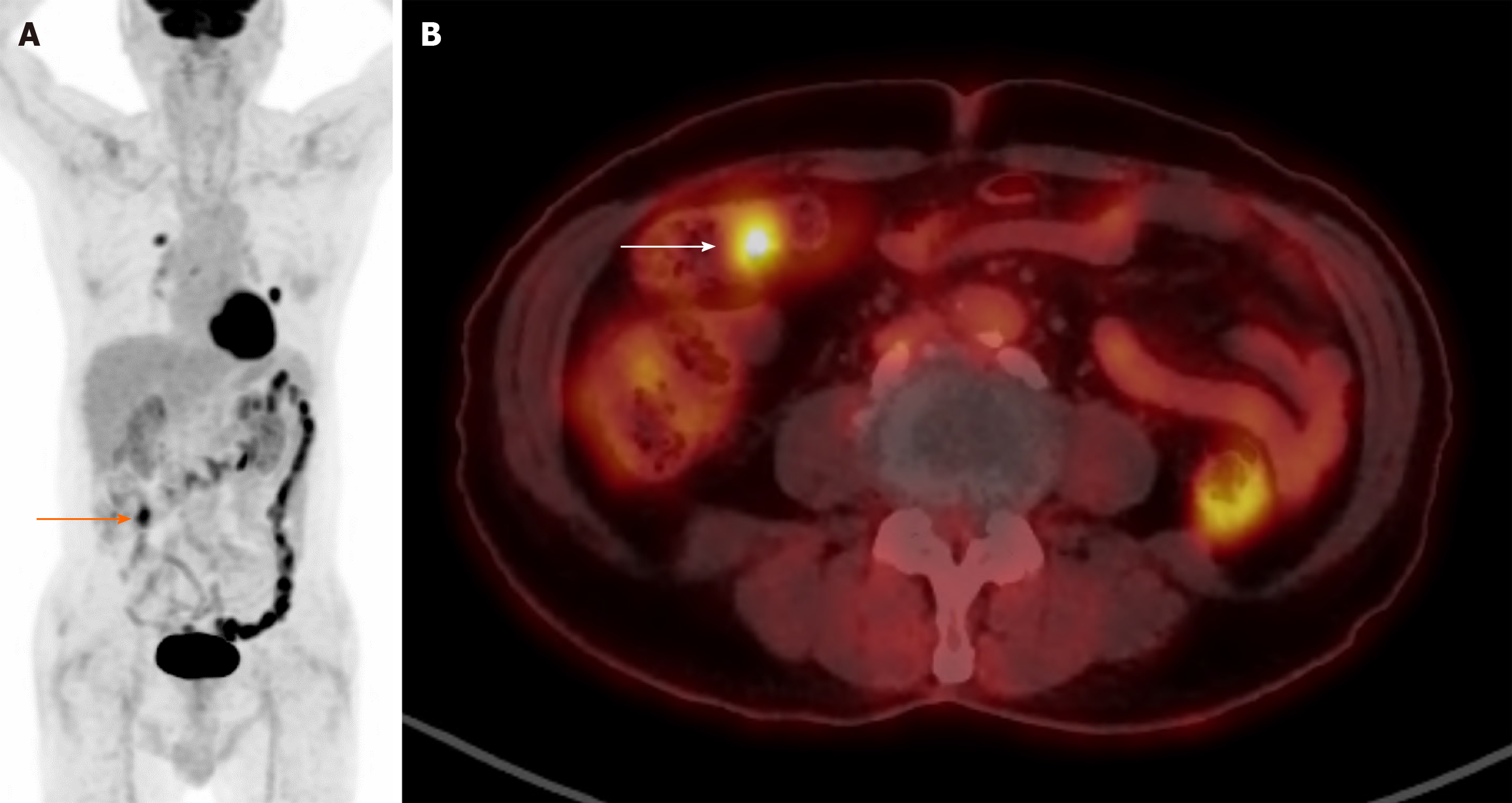
Figure 1 illustrates two cases of heightened FDG uptake in the gastrointestinal tract. In panel A, we observe diffuse intestinal FDG uptake in a 58-year-old male patient recently diagnosed with B-cell lymphoblastic lymphoma. Meanwhile, panel B presents hypermetabolism localized to the hepatic flexure area and descending colon of a 51-year-old woman with a previous history of gastric cancer. Despite the high metabolic activity, colonoscopic examinations in both cases revealed no pathological findings such as tumors or inflammation. This absence of detectable abnormalities underscores the physiological nature of the observed bowel uptake, suggesting the complexities and subtleties of interpreting such imaging findings. Figure 2 illustrates the distinct patterns of FDG uptake within the intestinal tract of an 87-year-old male patient diagnosed with non-small cell lung cancer. The image depicts both focal and diffuse FDG uptakes, which are crucial indicators in oncological imaging. Diffuse FDG uptake along the intestinal tract is commonly observed and often attributed to physiological processes. However, it is still imperative to discern between physiological and pathological uptake to guide clinical decision-making effectively. Of particular interest is the focal hypermetabolism detected in the proximal transverse colon. Such localized hypermetabolic activity raises concerns regarding the presence of a potential pathological lesion. In this case, subsequent colonoscopy was performed to elucidate the nature of the observed focal hypermetabolism. Despite the heightened suspicion, no significant abnormal lesions were identified during the procedure. These instances underscore the importance of discerning between physiological and pathological causes of increased FDG uptake in the gastrointestinal tract, particularly in the context of oncological surveillance.
F-18 FDG PET/CT, an integral component in the assessment of malignant diseases, often reveals suspicious focal colorectal FDG uptake. It is imperative to recognize that the technique has limitations, particularly in detecting non-FDG-avid or small malignant lesions. The likelihood of malignancy or advanced disease correlates with higher FDG uptake, underscoring the significance of diligent examination and interpretation of observed uptake.
Incidental colorectal FDG uptake occurs in approximately 5% of cases[76-78], with focal uptake presenting a higher likelihood of malignancy compared to diffuse patterns[71]. Diffuse and segmental uptake may stem from inflammation, physiological processes, or FDG excretion[79,80], generally associated with a lower risk of malignancy. Focal uptake prevalence reaches up to 16%[81], with malignant and premalignant lesions constituting around 70% of focal uptake with SUVmax exceeding approximately 5[72,81-85]. Colonoscopy is often recommended for further investigation in such cases[72,80,81,84-87], although ongoing debates surround the optimal PET parameters for distinguishing premalignant/malignant lesions from benign ones, with varying studies utilizing SUVmax[72,81,83,88,89].
Premalignant lesions are not yet malignant; however, they have more chance to develop into malignant lesions. Adenomas (tubular adenomas, villous adenomas, tubulovillous adenomas) are the most frequent premalignant lesions and others include chronic inflammatory bowel diseases, hereditary syndromes (familial adenomatous polyposis, Peutz-Jeghers syndrome, and juvenile polyposis). Colorectal adenomatous polyps are known to develop in up to 40% of people over the age of 60[90]. While debates persist regarding the role of SUV in differentiating malignant/premalignant from benign lesions[76,91-95], the significance of more than 50% malignancy in focal FDG colorectal uptake is noteworthy. In terms of cancer development by the location (distal or proximal), different genetic mechanisms are suggested[96-98], however, no significant difference was observed in SUVmax depending on location[34]. Notably, the observation of mixed single/multiple focal and diffuse uptake in the colorectal region underscores the complexity of interpretation. In such cases, diffuse uptake does not conclusively exclude the need for further evaluation, including colonoscopy and histopathological confirmation.
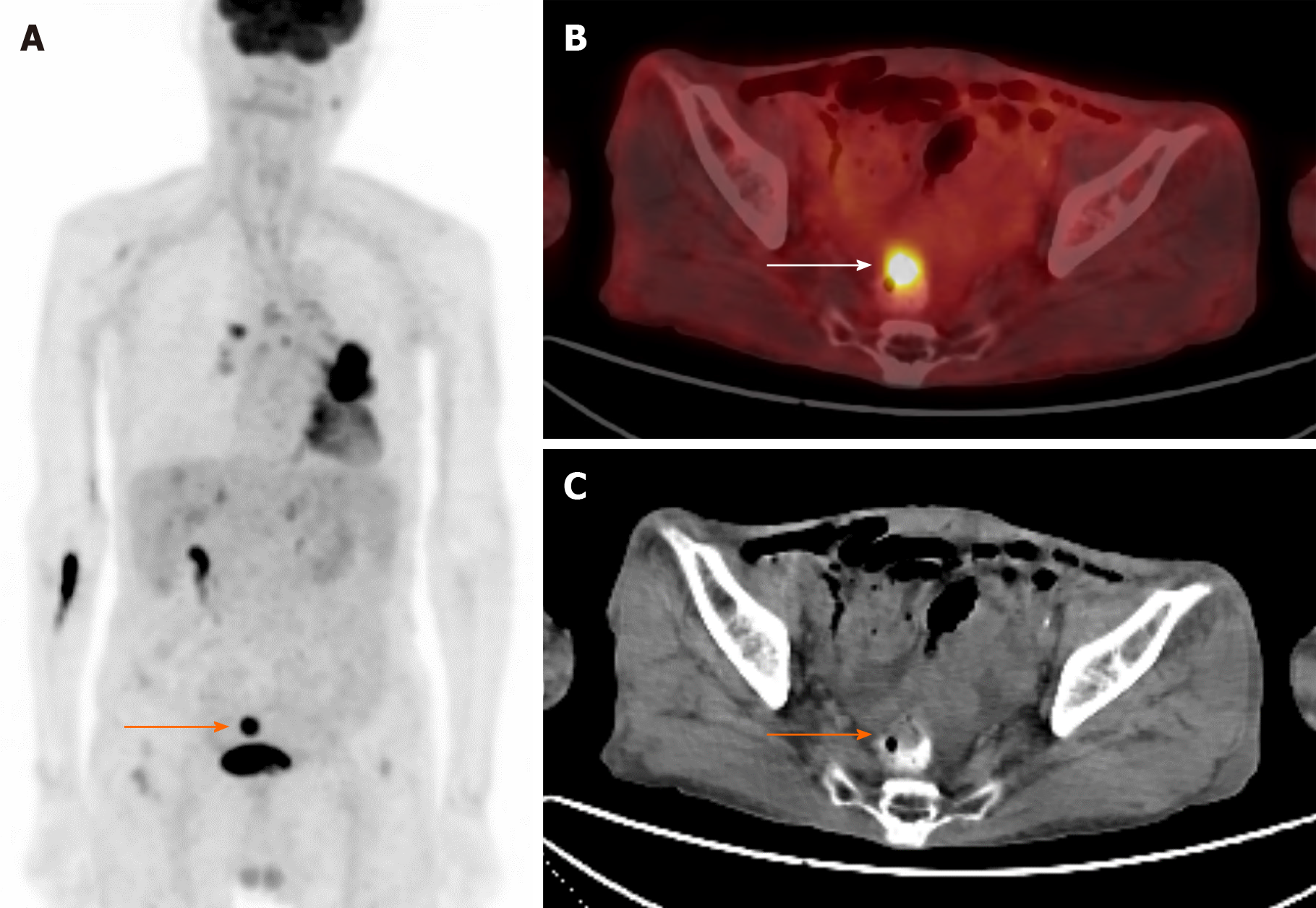
Figure 3 illustrates focal hypermetabolism incidentally detected in the rectum of an 88-year-old man previously diagnosed with non-small cell lung cancer in his left lung. Subsequent colonoscopy and pathological analysis confirmed the presence of adenocarcinoma originating from the rectum, establishing a secondary malignancy. This case highlights the importance of vigilant surveillance in cancer patients, as the detection of secondary malignancies or metastases can significantly impact treatment strategies, overall survival rates, and quality of life for patients.
The detection of unexpected focal colorectal F-18 FDG uptake is a frequent occurrence, with significant implications for clinical practice. Given the distinctive characteristics of FDG, notably its increased uptake in premalignant and malignant lesions, particularly when indicated by high SUV values, such findings warrant careful consideration. Studies have shown that a substantial proportion, approximately 70%, of focal colorectal FDG uptake corresponds to malignancies or premalignant lesions, especially when the SUVmax exceeds a threshold of approximately 5. While acknowledging the inherent limitations of F-18 FDG PET/CT, which may introduce uncertainty and hesitancy in interpretation, it is crucial to emphasize the diagnostic utility of observed FDG uptake patterns. Clinicians should be guided by these findings to pursue further proactive evaluation, thereby enhancing diagnostic precision and facilitating timely interventions. By leveraging the observed rates of malignancy and the degree of FDG uptake, clinicians can make informed decisions that optimize patient care and outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine & medical imaging
Country/Territory of origin: South Korea
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Sun XG, China S-Editor: Zheng XM L-Editor: A P-Editor: Zheng XM
| 1. | Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol. 2011;38:55-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Wong CY, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29:815-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Pacák J, Točík Z, Černý M. Synthesis of 2-deoxy-2-fluoro-D-glucose. J Chem Soc Chem Commun. 1969;77. [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Ido T, Wan CN, Casella V, Fowler JS, Wolf AP, Reivich M, Kuhl DE. Labeled 2-deoxy-D-glucose analogs. 18F-labeled 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2-fluoro-D-mannose and 14C-2-deoxy-2-fluoro-D-glucose. J Labelled Comp Radiopharm. 1978;14:175-183. [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Guerrero TM, Hoffman EJ, Dahlbom M, Cutler PD, Hawkins RA, Phelps ME. Characterization of a Whole-Body Imaging Technique for Pet. IEEE Trans Nucl Sci. 1990;37:676-680. [DOI] [Cited in This Article: ] |
| 6. | Cherry SR. Fundamentals of positron emission tomography and applications in preclinical drug development. J Clin Pharmacol. 2001;41:482-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Yu S. Review of F-FDG Synthesis and Quality Control. Biomed Imaging Interv J. 2006;2:e57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O'Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785-2808. [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Smith TA. Facilitative glucose transporter expression in human cancer tissue. Br J Biomed Sci. 1999;56:285-292. [PubMed] [Cited in This Article: ] |
| 10. | Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19:1154-1161. [PubMed] [Cited in This Article: ] |
| 11. | Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12:1131-1137. [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 165] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Rajendran JG, Mankoff DA, O'Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Coleman CN, Mitchell JB, Camphausen K. Tumor hypoxia: chicken, egg, or a piece of the farm? J Clin Oncol. 2002;20:610-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Annibaldi A, Widmann C. Glucose metabolism in cancer cells. Curr Opin Clin Nutr Metab Care. 2010;13:466-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Smith TA. FDG uptake, tumour characteristics and response to therapy: a review. Nucl Med Commun. 1998;19:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ; European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2053] [Cited by in F6Publishing: 1924] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 17. | Vangu MDT, Momodu JI. F-18 FDG PET/CT Imaging in Normal Variants, Pitfalls and Artifacts in the Abdomen and Pelvis. Front Nucl Med. 2022;1. [DOI] [Cited in This Article: ] |
| 18. | Li Y, Behr S. Acute Findings on FDG PET/CT: Key Imaging Features and How to Differentiate Them from Malignancy. Curr Radiol Rep. 2020;8:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Buyukdereli G, Kara E, Guler M, Kanat N. Evaluation of Visible Physiological F-18 FDG Uptake Patterns in Spinal Cord on PET/CT. Neurosurg Quart. 2015;25:403-406. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Purohit BS, Ailianou A, Dulguerov N, Becker CD, Ratib O, Becker M. FDG-PET/CT pitfalls in oncological head and neck imaging. Insights Imaging. 2014;5:585-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Kostakoglu L, Hardoff R, Mirtcheva R, Goldsmith SJ. PET-CT fusion imaging in differentiating physiologic from pathologic FDG uptake. Radiographics. 2004;24:1411-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 470] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519-1527. [PubMed] [Cited in This Article: ] |
| 24. | Nakamoto Y, Zasadny KR, Minn H, Wahl RL. Reproducibility of common semi-quantitative parameters for evaluating lung cancer glucose metabolism with positron emission tomography using 2-deoxy-2-[18F]fluoro-D-glucose. Mol Imaging Biol. 2002;4:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Krak NC, Boellaard R, Hoekstra OS, Twisk JW, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32:294-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med. 2012;53:4-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Oh HH, Lee SE, Choi IS, Choi WJ, Yoon DS, Min HS, Ra YM, Moon JI, Kang YH. The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol. 2011;104:530-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, van Lanschot J, Sloof G, Boellaard R. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2007;34:392-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Sung Y, Tetrault MA, Takahashi K, Ouyang J, Pratx G, Fakhri GE, Normandin MD. Dependence of fluorodeoxyglucose (FDG) uptake on cell cycle and dry mass: a single-cell study using a multi-modal radiography platform. Sci Rep. 2020;10:4280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Roppongi M, Izumisawa M, Terasaki K, Muraki Y, Shozushima M. (18)F-FDG and (11)C-choline uptake in proliferating tumor cells is dependent on the cell cycle in vitro. Ann Nucl Med. 2019;33:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Türkcan S, Kiru L, Naczynski DJ, Sasportas LS, Pratx G. Lactic Acid Accumulation in the Tumor Microenvironment Suppresses (18)F-FDG Uptake. Cancer Res. 2019;79:410-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, Aime S. In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res. 2016;76:6463-6470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | Li D, Wang Y, Liu W, Chen Q, Cai L, Xing X, Gao S. The Correlation between (18)F-FDG PET/CT Imaging SUVmax of Preoperative Colon Cancer Primary Lesions and Clinicopathological Factors. J Oncol. 2021;2021:4312296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Verger A, Imbert L, Zaragori T. Dynamic amino-acid PET in neuro-oncology: a prognostic tool becomes essential. Eur J Nucl Med Mol Imaging. 2021;48:4129-4132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Najjar AM, Johnson JM, Schellingerhout D. The Emerging Role of Amino Acid PET in Neuro-Oncology. Bioengineering (Basel). 2018;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Goldstein J, Even-Sapir E, Ben-Haim S, Saad A, Spieler B, Davidson T, Berger R, Weiss I, Appel S, Lawrence YR, Symon Z. Does Choline PET/CT Change the Management of Prostate Cancer Patients With Biochemical Failure? Am J Clin Oncol. 2017;40:256-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Urso L, Rocca GC, Borgia F, Lancia F, Malorgio A, Gagliano M, Zanetto M, Uccelli L, Cittanti C, Ippolito C, Evangelista L, Bartolomei M. The Role of [(18)F]F-Choline PET/CT in the Initial Management and Outcome Prediction of Prostate Cancer: A Real-World Experience from a Multidisciplinary Approach. Biomedicines. 2022;10. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 39. | Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology. 1992;183:643-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med. 1993;34:1-6. [PubMed] [Cited in This Article: ] |
| 41. | Minn H, Leskinen-Kallio S, Lindholm P, Bergman J, Ruotsalainen U, Teräs M, Haaparanta M. [18F]fluorodeoxyglucose uptake in tumors: kinetic vs. steady-state methods with reference to plasma insulin. J Comput Assist Tomogr. 1993;17:115-123. [PubMed] [Cited in This Article: ] |
| 42. | Langen KJ, Braun U, Rota Kops E, Herzog H, Kuwert T, Nebeling B, Feinendegen LE. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med. 1993;34:355-359. [PubMed] [Cited in This Article: ] |
| 43. | Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, Stabin MG, Zubal G, Kachelriess M, Cronin V, Holbrook S. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885-895. [PubMed] [Cited in This Article: ] |
| 44. | Hara T, Higashi T, Nakamoto Y, Suga T, Saga T, Ishimori T, Ishizu K, Kawashima H, Kawase S, Matsumoto K, Togashi K. Significance of chronic marked hyperglycemia on FDG-PET: is it really problematic for clinical oncologic imaging? Ann Nucl Med. 2009;23:657-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Lindholm H, Brolin F, Jonsson C, Jacobsson H. The relation between the blood glucose level and the FDG uptake of tissues at normal PET examinations. EJNMMI Res. 2013;3:50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Sprinz C, Altmayer S, Zanon M, Watte G, Irion K, Marchiori E, Hochhegger B. Effects of blood glucose level on 18F-FDG uptake for PET/CT in normal organs: A systematic review. PLoS One. 2018;13:e0193140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Eskian M, Alavi A, Khorasanizadeh M, Viglianti BL, Jacobsson H, Barwick TD, Meysamie A, Yi SK, Iwano S, Bybel B, Caobelli F, Lococo F, Gea J, Sancho-Muñoz A, Schildt J, Tatcı E, Lapa C, Keramida G, Peters M, Boktor RR, John J, Pitman AG, Mazurek T, Rezaei N. Effect of blood glucose level on standardized uptake value (SUV) in (18)F- FDG PET-scan: a systematic review and meta-analysis of 20,807 individual SUV measurements. Eur J Nucl Med Mol Imaging. 2019;46:224-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 48. | Cengİz A. The Relation Between the Blood Glucose Level and the FDG Uptake of Tissues at Normal or Near-Normal PET/CT Imaging. Akd Med J. 2019;5:365-369. [DOI] [Cited in This Article: ] |
| 49. | Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 584] [Cited by in F6Publishing: 532] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 50. | Czernin J. Clinical applications of FDG-PET in oncology. Acta Med Austriaca. 2002;29:162-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Pinilla I, Rodríguez-Vigil B, Gómez-León N. Integrated FDG PET/CT: Utility and Applications in Clinical Oncology. Clin Med Oncol. 2008;2:181-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Liu T, Behr S, Khan S, Osterhoff R, Aparici CM. Focal Colonic FDG Activity with PET/CT: Guidelines for Recommendation of Colonoscopy. World J Nucl Med. 2015;14:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Cook GJ, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Eur J Nucl Med. 1999;26:1363-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19:61-77; quiz 150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 624] [Cited by in F6Publishing: 546] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 55. | Cook GJ, Fogelman I, Maisey MN. Normal physiological and benign pathological variants of 18-fluoro-2-deoxyglucose positron-emission tomography scanning: potential for error in interpretation. Semin Nucl Med. 1996;26:308-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 291] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 56. | Ahmad Sarji S. Physiological uptake in FDG PET simulating disease. Biomed Imaging Interv J. 2006;2:e59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol. 2012;198:W304-W314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 58. | Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972-1980. [PubMed] [Cited in This Article: ] |
| 59. | Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551-1555. [PubMed] [Cited in This Article: ] |
| 60. | Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 61. | Safaie E, Matthews R, Bergamaschi R. PET scan findings can be false positive. Tech Coloproctol. 2015;19:329-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation--current and emerging clinical applications. Clin Radiol. 2015;70:787-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 63. | Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, Mantzarides M, Foehrenbach H, Pecking AP, Alberini JL. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging. 2008;35:95-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 64. | Oh JR, Song HC, Chong A, Ha JM, Jeong SY, Min JJ, Bom HS. Impact of medication discontinuation on increased intestinal FDG accumulation in diabetic patients treated with metformin. AJR Am J Roentgenol. 2010;195:1404-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Ozülker T, Ozülker F, Mert M, Ozpaçaci T. Clearance of the high intestinal (18)F-FDG uptake associated with metformin after stopping the drug. Eur J Nucl Med Mol Imaging. 2010;37:1011-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Bybel B, Greenberg ID, Paterson J, Ducharme J, Leslie WD. Increased F-18 FDG intestinal uptake in diabetic patients on metformin: a matched case-control analysis. Clin Nucl Med. 2011;36:452-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Steenkamp DW, McDonnell ME, Meibom S. Metformin may be associated with false-negative cancer detection in the gastrointestinal tract on PET/CT. Endocr Pract. 2014;20:1079-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. Radiographics. 2007;27:145-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Tatlidil R, Jadvar H, Bading JR, Conti PS. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology. 2002;224:783-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 70. | Kresnik E, Mikosch P, Gallowitsch HJ, Heinisch M, Lind P. F-18 fluorodeoxyglucose positron emission tomography in the diagnosis of inflammatory bowel disease. Clin Nucl Med. 2001;26:867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Shmidt E, Nehra V, Lowe V, Oxentenko AS. Clinical significance of incidental [18 F]FDG uptake in the gastrointestinal tract on PET/CT imaging: a retrospective cohort study. BMC Gastroenterol. 2016;16:125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Gökden Y, Özülker F, Özülker T. Prevalence and Clinical Significance of Incidental Focal (18)F-FDG Uptake in Colon on PET/CT Imaging. Mol Imaging Radionucl Ther. 2022;31:96-103. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 73. | de Leijer JF, Metman MJH, van der Hoorn A, Brouwers AH, Kruijff S, van Hemel BM, Links TP, Westerlaan HE. Focal Thyroid Incidentalomas on (18)F-FDG PET/CT: A Systematic Review and Meta-Analysis on Prevalence, Risk of Malignancy and Inconclusive Fine Needle Aspiration. Front Endocrinol (Lausanne). 2021;12:723394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 74. | Aarstad EM, Nordhaug P, Naghavi-Behzad M, Larsen LB, Gerke O, Hildebrandt MG. Prevalence of focal incidental breast uptake on FDG-PET/CT and risk of malignancy: a systematic review and meta-analysis. Eur J Hybrid Imaging. 2019;3:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Brown AM, Lindenberg ML, Sankineni S, Shih JH, Johnson LM, Pruthy S, Kurdziel KA, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B. Does focal incidental 18F-FDG PET/CT uptake in the prostate have significance? Abdom Imaging. 2015;40:3222-3229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | van Hoeij FB, Keijsers RG, Loffeld BC, Dun G, Stadhouders PH, Weusten BL. Incidental colonic focal FDG uptake on PET/CT: can the maximum standardized uptake value (SUVmax) guide us in the timing of colonoscopy? Eur J Nucl Med Mol Imaging. 2015;42:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Keyzer C, Dhaene B, Blocklet D, De Maertelaer V, Goldman S, Gevenois PA. Colonoscopic Findings in Patients With Incidental Colonic Focal FDG Uptake. AJR Am J Roentgenol. 2015;204:W586-W591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Hui YH, Kung BT, Yong TKA. Incidental Focal Colonic Uptake of 18F-fluorodeoxyglucose on Positron Emission Tomography/Computed Tomography: Its Incidence and Clinical Significance. Hong Kong J Radiol. 2020;23:275-280. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Şimşek FS, İspiroğlu M, Taşdemir B, Köroğlu R, Ünal K, Özercan IH, Entok E, Kuşlu D, Karabulut K. What approach should we take for the incidental finding of increased 18F-FDG uptake foci in the colon on PET/CT? Nucl Med Commun. 2015;36:1195-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Salazar Andía G, Prieto Soriano A, Ortega Candil A, Cabrera Martín MN, González Roiz C, Ortiz Zapata JJ, Cardona Arboniés J, Lapeña Gutiérrez L, Carreras Delgado JL. Clinical relevance of incidental finding of focal uptakes in the colon during 18F-FDG PET/CT studies in oncology patients without known colorectal carcinoma and evaluation of the impact on management. Rev Esp Med Nucl Imagen Mol. 2012;31:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Treglia G, Taralli S, Salsano M, Muoio B, Sadeghi R, Giovanella L. Prevalence and malignancy risk of focal colorectal incidental uptake detected by (18)F-FDG-PET or PET/CT: a meta-analysis. Radiol Oncol. 2014;48:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Albertsen LN, Jaensch C, Tornbjerg SM, Teil J, Madsen AH. Correlation between incidental focal colorectal FDG uptake on PET/CT and colonoscopic and histopathological results. Scand J Gastroenterol. 2022;57:246-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Xu W, Li H, Guo Z, Zhang L, Zhang R. Combined SUVmax and localized colonic wall thickening parameters to identify high-risk lesions from incidental focal colorectal (18)F-FDG uptake foci. Front Oncol. 2022;12:972096. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 84. | Kousgaard SJ, Gade M, Petersen LJ, Thorlacius-Ussing O. Incidental detection of colorectal lesions on (18) F-FDG-PET/CT is associated with high proportion of malignancy: A study in 549 patients. Endosc Int Open. 2020;8:E1725-E1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Fuertes J, Montagut C, Bullich S, Coma MI, Mestre-Fusco A, Suárez-Piñera M, Trampal C, Bellmunt J. Incidental focal uptake in colorectal location on oncologic ¹⁸FDG PET and PET/CT studies: histopathological findings and clinical significances. Rev Esp Med Nucl Imagen Mol. 2015;34:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Rigault E, Lenoir L, Bouguen G, Pagenault M, Lièvre A, Garin E, Siproudhis L, Bretagne JF. Incidental colorectal focal (18) F-FDG uptake: a novel indication for colonoscopy. Endosc Int Open. 2017;5:E924-E930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Lee C, Koh SJ, Kim JW, Lee KL, Im JP, Kim SG, Kim JS, Jung HC, Kim BG. Incidental colonic 18F-fluorodeoxyglucose uptake: do we need colonoscopy for patients with focal uptake confined to the left-sided colon? Dig Dis Sci. 2013;58:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Cho SH, Kim SW, Kim WC, Park JM, Yoo IeR, Kim SH, Oh ST. Incidental focal colorectal ¹⁸F-fluorodeoxyglucose uptake on positron emission tomography/computed tomography. World J Gastroenterol. 2013;19:3453-3458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Wu GZ, Sun D, Chen JY, Qiu JM, Kong Y. [Clinical diagnostic value of (18)F-FDG PET-CT in incidental finding of focal hypermetabolism focus in the colon and rectum]. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:555-560. [PubMed] [Cited in This Article: ] |
| 90. | Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551-2557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 91. | Servente L, Gigirey V, García Fontes M, Alonso O. Incidental focal colonic uptake in studies (18)F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2018;37:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 92. | Soltau SR, Hess S, Nguyen T, Gerke O, Petersen H, Alavi A, Høilund-Carlsen PF. Clinical significance of incidental focal bowel uptake on (18)F-FDG PET/CT as related to colorectal cancer. Hell J Nucl Med. 2016;19:245-249. [PubMed] [Cited in This Article: ] |
| 93. | Purandare NC, Gawade SK, Puranik AD, Agrawal A, Shah S, Rangarajan V. Etiology and significance of incidentally detected focal colonic uptake on FDG PET/CT. Indian J Radiol Imaging. 2012;22:260-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Kei PL, Vikram R, Yeung HW, Stroehlein JR, Macapinlac HA. Incidental finding of focal FDG uptake in the bowel during PET/CT: CT features and correlation with histopathologic results. AJR Am J Roentgenol. 2010;194:W401-W406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Weston BR, Iyer RB, Qiao W, Lee JH, Bresalier RS, Ross WA. Ability of integrated positron emission and computed tomography to detect significant colonic pathology: the experience of a tertiary cancer center. Cancer. 2010;116:1454-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 531] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 97. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8087] [Cited by in F6Publishing: 7736] [Article Influence: 227.5] [Reference Citation Analysis (1)] |
| 98. | Beart RW, Melton LJ 3rd, Maruta M, Dockerty MB, Frydenberg HB, O'Fallon WM. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983;26:393-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 3.3] [Reference Citation Analysis (0)] |