Published online Nov 16, 2023. doi: 10.12998/wjcc.v11.i32.7926
Peer-review started: September 26, 2023
First decision: October 9, 2023
Revised: October 23, 2023
Accepted: November 9, 2023
Article in press: November 9, 2023
Published online: November 16, 2023
Several reports of adult-onset immunodeficiency syndrome have been associated with anti-interferon-gamma (IFN-γ) autoantibodies (AIGAs). However, it is rare to find AIGAs with intracranial infections.
In this case study, we report a case of an AIGAs with intracranial infection and hand rashes considered Sweet’s syndrome. The patient presented to our hospital with a persistent cough, a fever that had been going on for 6 mo, and a rash that had been going on for a week. The patient started losing consciousness gradually on the fourth day after admission, with neck stiffness and weakened limb muscles. The upper lobe of the left lung had a high-density mass with no atypia and a few inflammatory cells in the interstitium. Brain magnetic resonance imaging and cerebrospinal fluid suggest intracranial infection. The pathology of the skin damage on the right upper extremity revealed an infectious lesion that was susceptible to Sweet’s disease. It has an anti-IFN-γ autoantibody titer of 1:2500. She was given empirical anti-non-tuberculous mycobacterial and anti
Adults with severe and recurrent infections of several organs should be considered for AIGAs if no other known risk factors exist. AIGAs are susceptible to subsequent intracranial infections and Sweet’s syndrome.
Core Tip: Anti-interferon-gamma (IFN-γ) autoantibodies (AIGAs) have been associated with adult-onset immunodeficiency syndrome. Most patients have multiple organ involvement upon presentation; lymph nodes are the most frequently affected organ, followed by the skin, lungs, bones, and joints. We describe a patient with AIGAs who also had an intracranial infection and hand rashes as Sweet’s syndrome. Anti-IFN-γ was increased despite the lack of a confirmed non-tuberculous mycobacterial (NTM) infection, and the empirical anti-NTM treatment in this patient was successful. Without recognized risk factors, AIGAs should be considered in patients with severe and recurrent infections of multiple organs. In AIGAs, subsequent intracranial infection and Sweet’s syndrome are possible.
- Citation: Zheng JH, Wu D, Guo XY. Intracranial infection accompanied sweet’s syndrome in a patient with anti-interferon-γ autoantibodies: A case report. World J Clin Cases 2023; 11(32): 7926-7934
- URL: https://www.wjgnet.com/2307-8960/full/v11/i32/7926.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i32.7926
Adult-onset immunodeficiency syndrome associated with anti-interferon-gamma (IFN-γ) autoantibodies (AIGAs) has been discovered in Southeast Asia, including Thailand, Vietnamese, Japan, the Hong Kong Special Administrative Region of China, and Taiwan, since it was first reported in the Philippines in 2004[1-7].
The strong correlation between high-titer neutralizing antibodies to IFN-γ and this adult-onset immunodeficiency syndrome supports the critical function of IFN-γ in controlling many pathogens. Autoantibodies against IFN-γ have been linked to opportunistic infections, most frequently non-tuberculous mycobacterial (NTM) infections, and others, and can lead to immunodeficiency[1,8,9]. Thus, AIGAs may be viewed as a new form of late-onset immunodeficiency that confers a predisposition to some bacterial and fungal infections and severe mycobacterial illnesses.
Most patients have multiple organ involvement upon presentation; lymph nodes are the most often affected organ, followed by the skin, lungs, bones, blood, and joints[10]. The non-specific symptoms and rarity of this syndrome make it challenging to identify in the early stages of the disease. According to reports, 49%-57% of AIGAs had skin manifestations[11,12]. The skin manifestation of adult-onset immunodeficiency syndrome, known as Sweet’s syndrome, is frequently associated with AIGAs[13]. Acute febrile neutrophilic dermatosis, another name for Sweet’s syndrome, is a rare inflammatory condition. Its symptoms are acute onset dermal neutrophilic lesions, leukocytosis, and pyrexia. Sweet’s syndrome frequently appears as erythematous plaques and nodules and is most commonly seen in reactive dermatitis. We have reported a patient with intracranial infection accompanied by hand rashes who also had IFN-γ autoantibodies, a condition known as Sweet's syndrome.
A 51-year-old female came to our hospital for treatment and presented with a persistent cough, fever for 6 mo, and rash for a week.
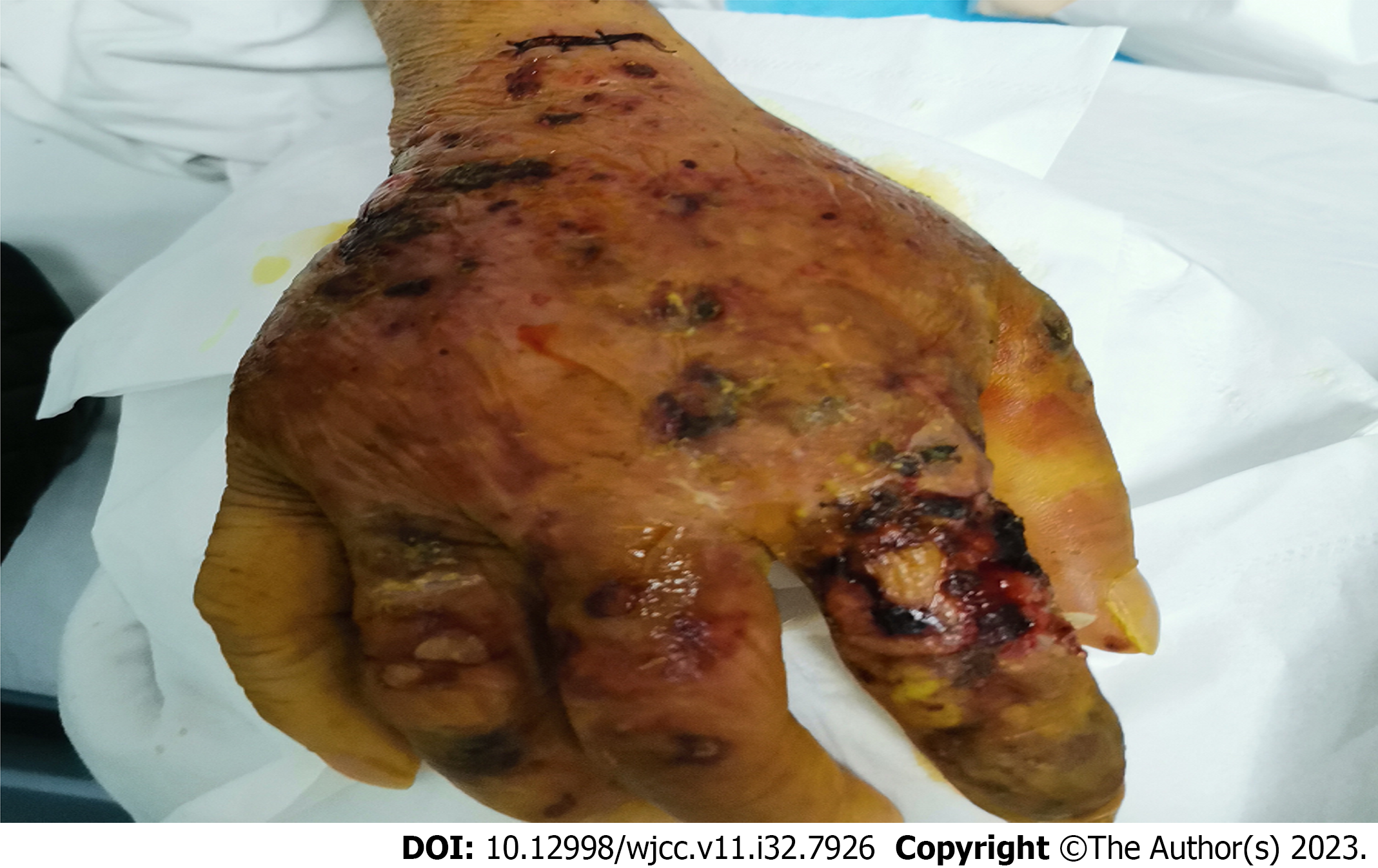
Symptoms started 6 mo back with recurrent cough and fever. Additionally, the lymph nodes in her right neck were swollen. A cervical lymph node biopsy revealed lymph node tuberculosis from the outer hospital. The cough subsided, and the lymph nodes shrank after anti-tuberculosis therapy. However, the cough persisted and recurred. Although chest computed tomography (CT) enhancement showed exudation and proliferation of tuberculous lesions in the upper lobe of the left lung, neoplastic lesions were not ruled out. Several inflammatory cell infiltration and necrosis, which are changes brought on by infection, were observed in the pathological findings. According to the thoracoscopic (left pleural) view, the vessels and lymphatic vessels were hyperplastic and dilated in the fibrous tissue. A significant amount of lymphocytes, plasma cells, and neutrophils were infiltrated. There was mild hyperplasia of mesenchymal cells on the surface, demonstrating inflammatory alterations, and hyperplasia of local tissue cells, some of which were papillary and crystal in appearance. The cough improved after receiving anti-infection and anti-tuberculosis treatment. However, the cough persisted to some degree. A week prior, the fever returned, and a rash with partial blisters and ulceration, together with pus flow and blackness, emerged on the right upper limb (Figure 1). The body had multiple nodules, partial ulceration, and a slight exudation. A paroxysmal headache followed it. On the fourth day after being admitted, the patient started to gradually lose consciousness and experience neck stiffness and weakened limb muscles. The patient slowly developed lethargy.
The patient denied having ever had an infection, such as tuberculosis.
The patient denied having a history of tumors or similar diseases in her family.
The vital signs were as follows upon physical examination: body temperature, 36.4 ℃; blood pressure, 119/86 mmHg; heart rate, 109 beats per min; and respiratory rate, 20 breaths per min. A rash with partial blisters, ulceration, pus flow, and blackness appeared on the right upper limb. Many nodules and partial ulcerations were seen throughout the body, along with slight exudation. Four days after being admitted, the patient gradually showed signs of lethargy, neck resistance, grade IV muscle strength in both upper limbs, grade II muscle strength in both lower limbs, and a positive Babinski sign on both sides.
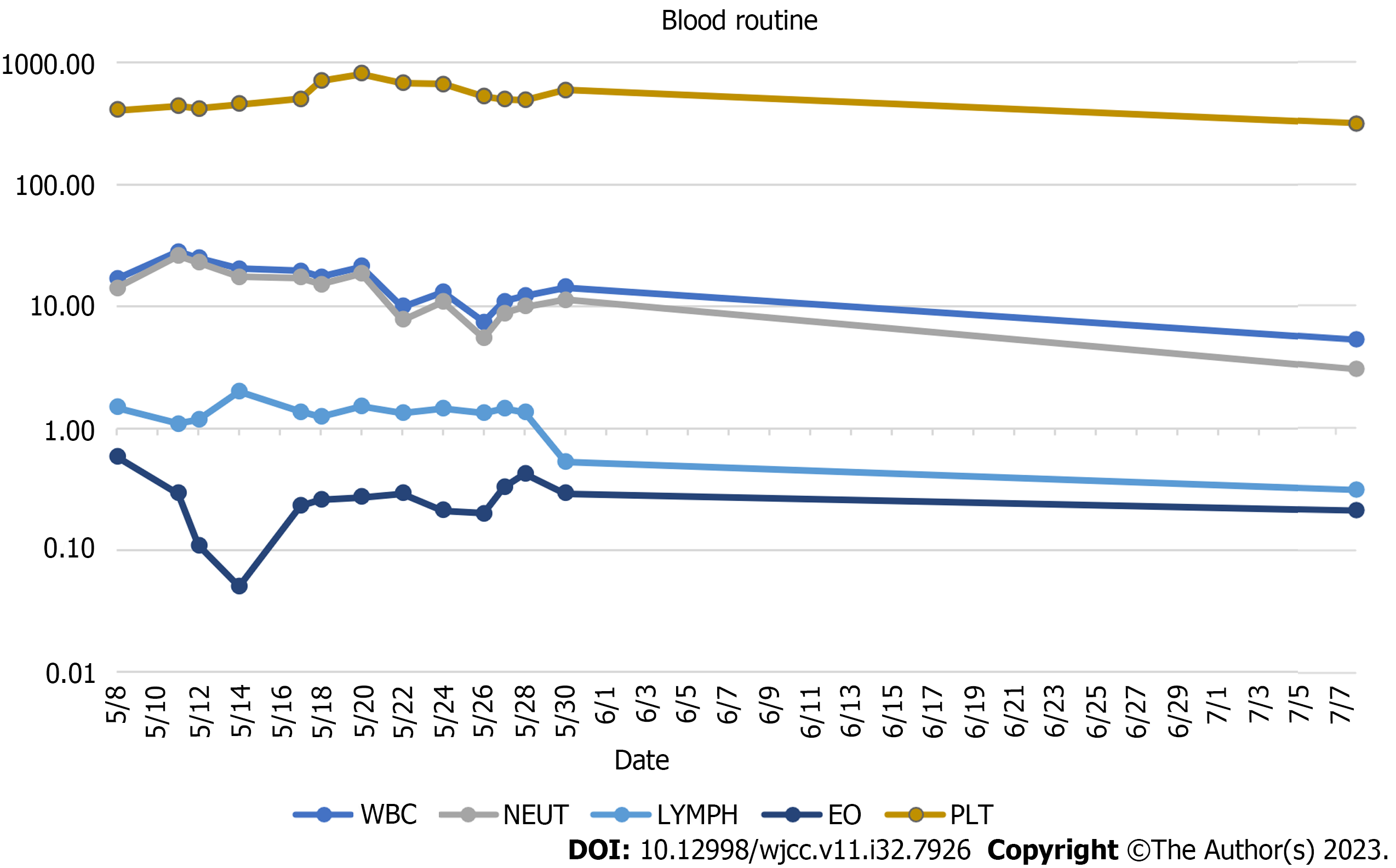
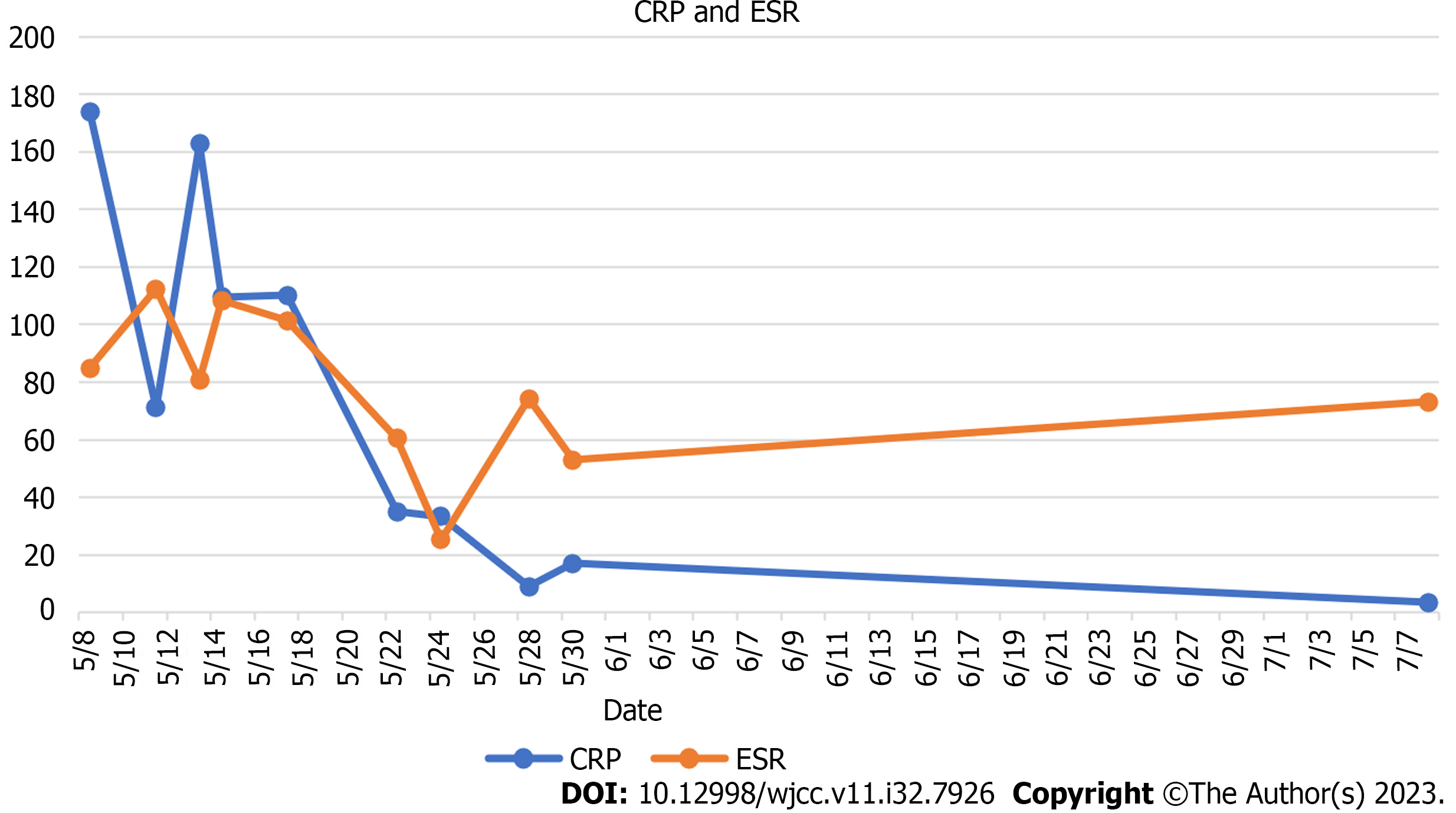
The peripheral white blood cell count was 16.9 × 109/L (3.69-16 × 109/L), ranging from 14.18 × 109/L neutrophils (2-7.7 × 109/L), 0.58 × 109/L eosinophilic (0.05-0.5 × 109/L), 1.48 × 109/L lymphocytes (0.8-4 × 109/L), and 405 × 109 /L (100-300 × 109/L) platelets (Figure 2) at the time of admission. Serum C-reactive protein concentration of 173.98 mg/L (0-3 mg/L) and erythrocyte sedimentation rate of 84.8 mm/h (0-20) were significant indicators of the inflammatory syndrome (Figure 3). The tumor marker had a carbohydrate antigen 125 level of 46 U/mL (≤ 35 U/mL). Humoral immunity was normal except for a slight increase in immunoglobulins G: 23.85 g/L (8-16 g/L). The number of inhibitory lymphocytes decreased by 9.3% (19%-48%) (Table 1). Antibodies to the human immunodeficiency virus are negative. Antibodies against tuberculobacter are positive. A bone marrow puncture was done due to increased levels of white blood cells and platelets. The results of the bone marrow aspiration showed reactive hyperplasia, no aberrant hyperplasia of juvenile cells, and no lymphoma.
| Before therapy (2021/5/9) | After treatment (2021/7/8) | |
| CA125 (≤ 35 U/mL) | 46 | 12.7 |
| IgG (8-16 g/L) | 23.85 | 17.91 |
| Th (19%-48%) | 9.3 | 18.7 |
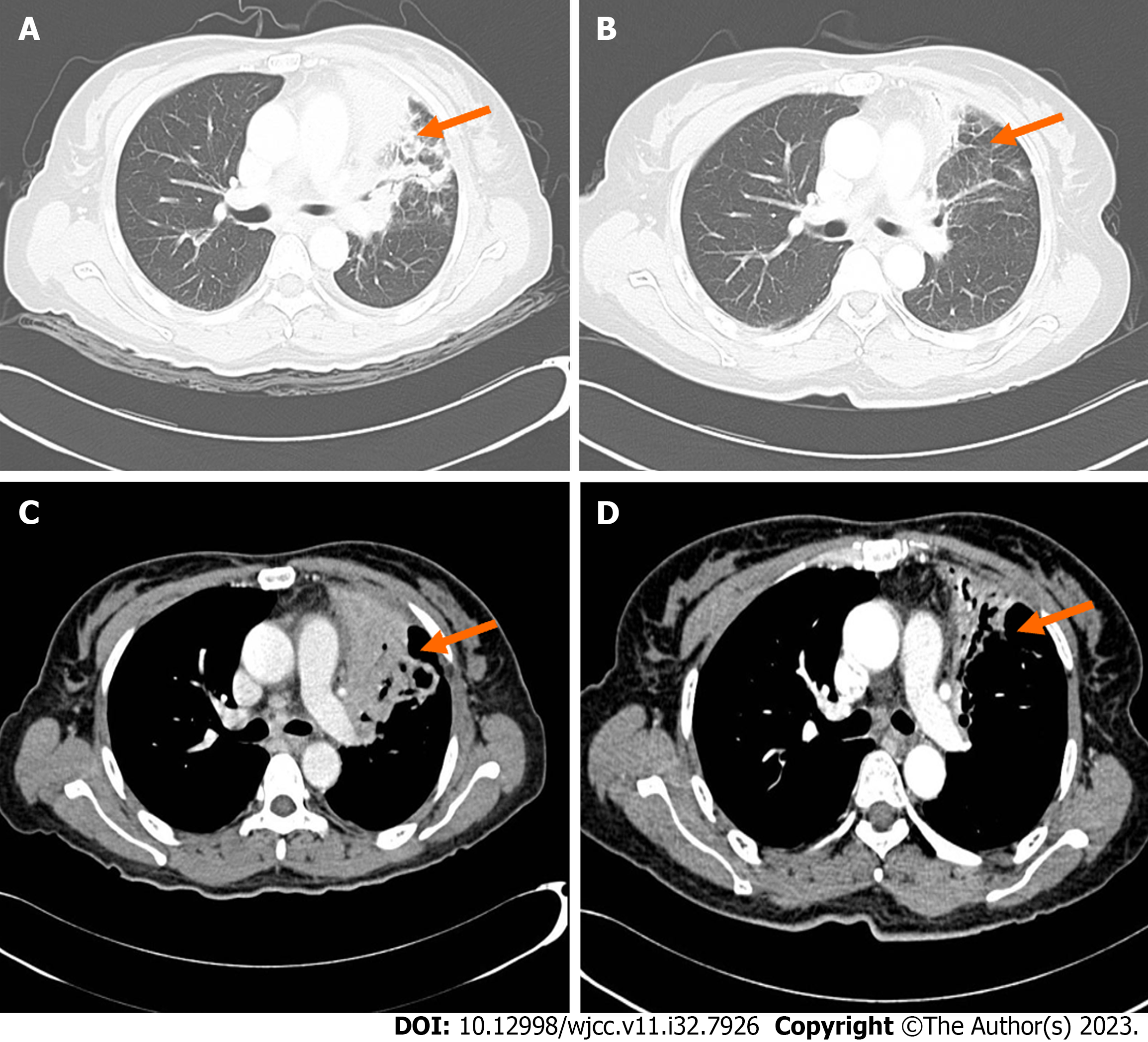
Chest CT improvement showed a 7.7 cm × 7.2 cm × 5.9 cm high-density mass in the left upper lobe of the left (Figure 4). There was no atypia and a small number of inflammatory cells in the interstitium, according to the pathological results of the left upper lobe of the lung. In alveolar lavage fluid, no malignant tumor cells were found. Yeast-like fungal spores were discovered in the pulmonary branch brush. The results of the second-generation sequencing of the lung tissue suggested Propionibacterium acnes.
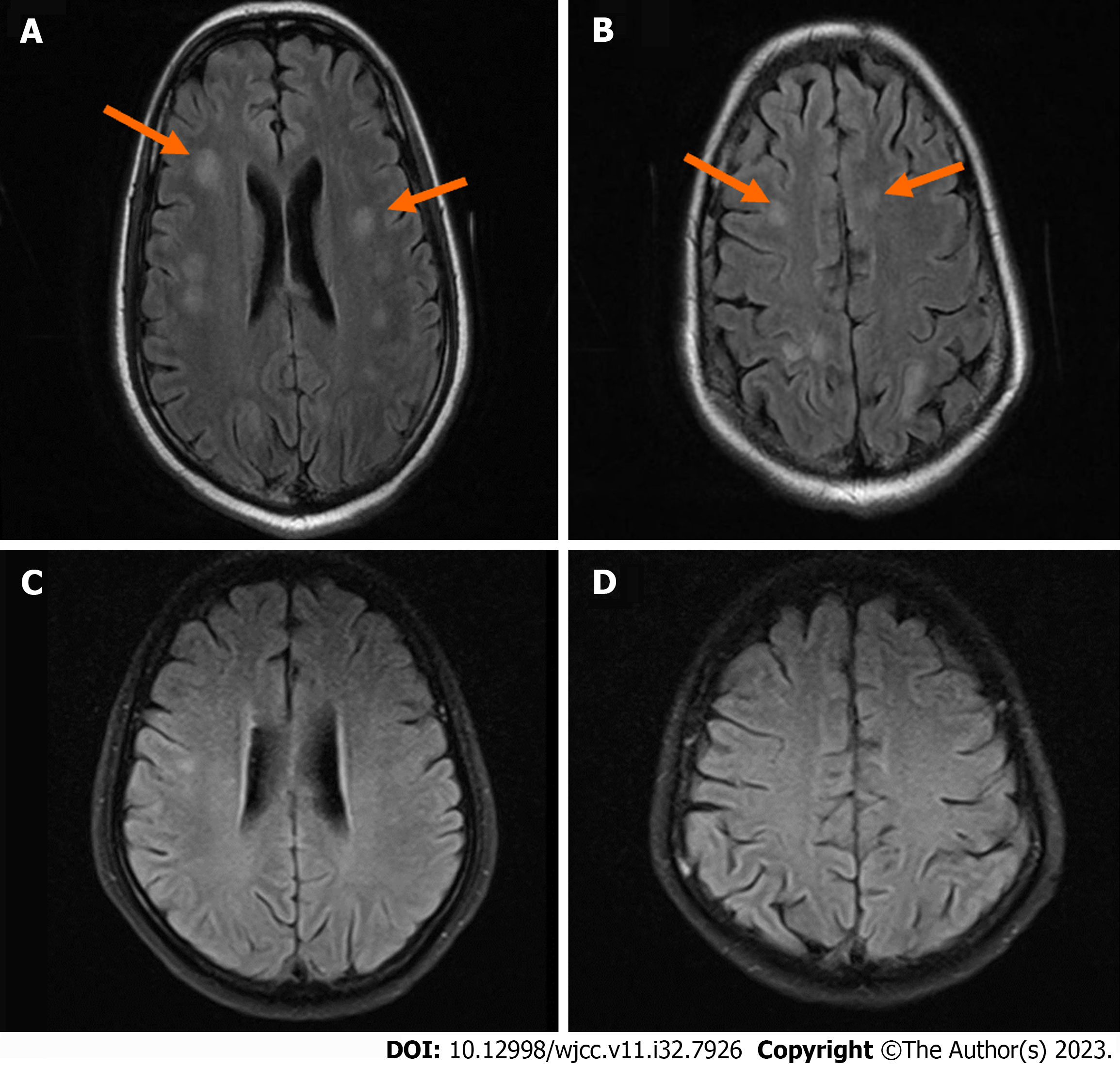
Multiple ischemia lesions were found in the right basal ganglia, bilateral frontal lobes, periventricular, radiative crown, and hemioval center on a brain magnetic resonance imaging (MRI) scan plus enhancement (Figure 5). The results of the CT angiography revealed no definitive stenosis, occlusion, dilation, or aberrant vascular signs in bilateral anterior, middle, and posterior cerebral arteries and bilateral internal carotid, basilar, and vertebral arteries. The patient had a lumbar puncture, and the cerebrospinal fluid (CSF) was examined. CSF was clear and colorless. The Pandy test was weakly positive. The total number of white blood cells in CSF was 166 × 106/L, with neutral lobule accounting for 76.1%, lymphocytes accounting for 23.6%, and acid cells accounting for 0.3%. The protein content of CSF was 0.56g/L, and the chlorine level in CSF was 118 mmol/L (Table 2). CSF-based section showed more neutrophils and no malignant tumor cells.
| CSF parameter | Normal | Before therapy (2021/5/11) | After treatment (2021/5/17) |
| CSF pressure (mmH2O) | 80-180 | 230 | 160 |
| Appearance | Clear | Clear | Clear |
| Pandy test | Negative | Weak positive | Weak positive |
| CSF WBC (106/L) | 0-5 | 166 | 15 |
| CSF N (%) | 76.1 | 17.9 | |
| CSF L (%) | 23.6 | 72 | |
| CSF E (%) | 0.3 | 0.1 | |
| CSF protein (g/L) | 0.08-0.45 | 0.56 | 0.72 |
| CSFchloride ion (mmol/L) | 120-130 | 118 | 113 |
| CSF glucose (mmol/L) | 2.5-4.4 | 2.66 | 2.48 |
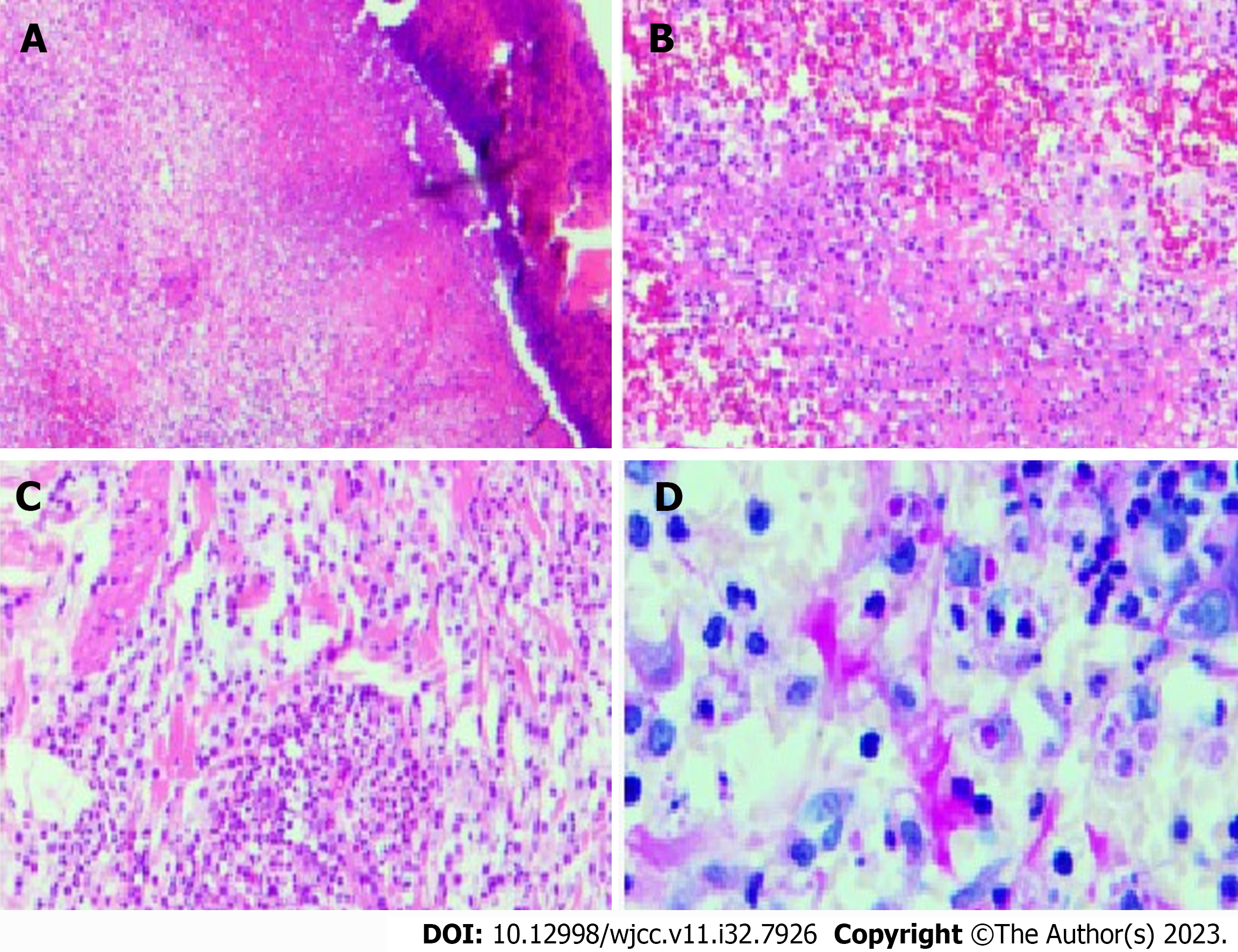
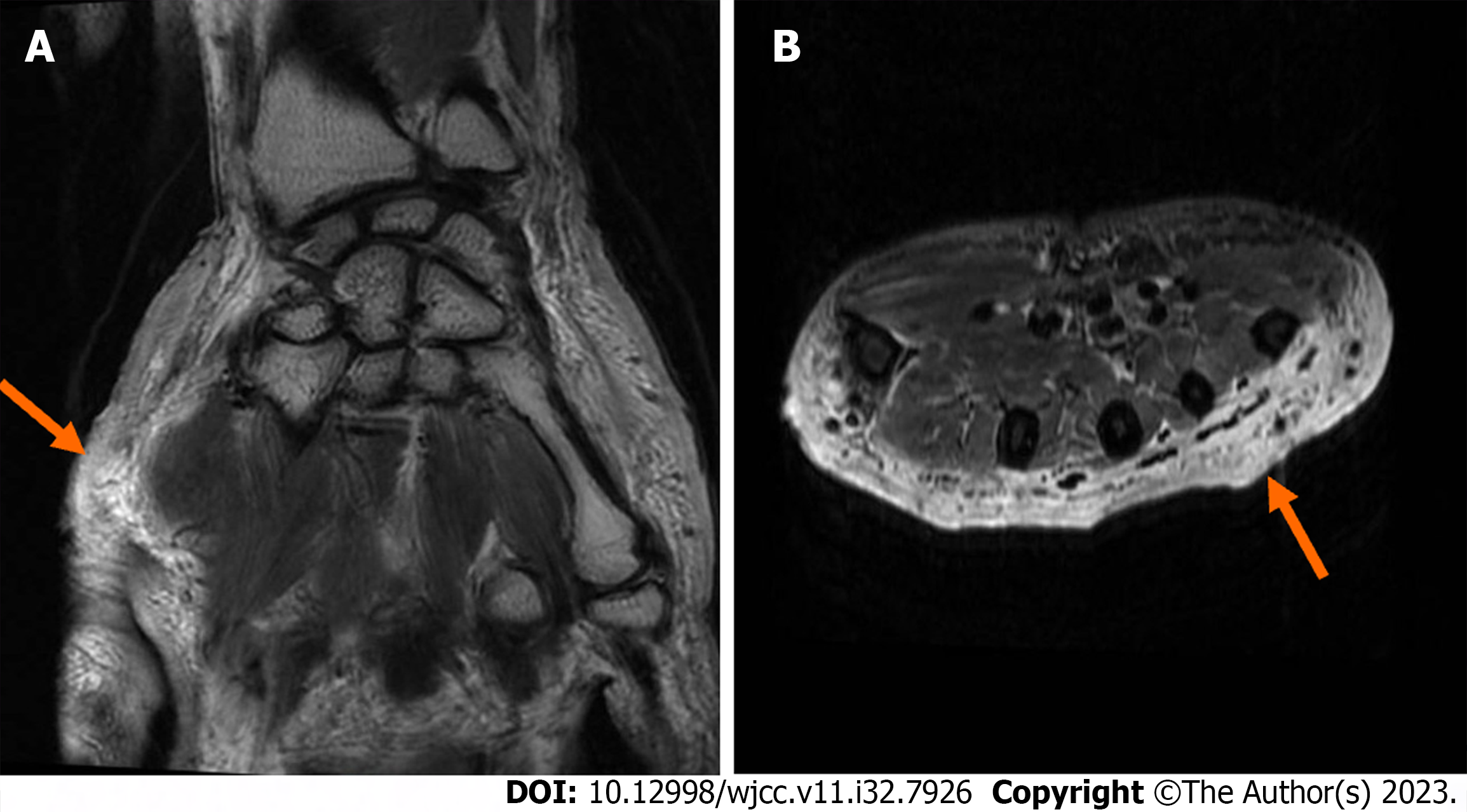
Fungi, tuberculosis, and bacteria were not discovered in the wound secretion of the right upper limb through several smears and cultures. According to the pathology of the damaged skin on the right upper extremity, Sweet disease-predisposed neutrophilic dermatitis was an infectious lesion (Figure 6). The antacid did not affect the specific discoloration. With no signs of lymphoma or tuberculosis, periodic acid-Schiff stains revealed eosinophilic bodies in the cytoplasm of histiocytes, which required differentiation from fungi. Skin sequencing from the second generation revealed Malassezia and Malassezia globoides. The MRI results of the right hand showed a slight swelling of the right hand, and the subcutaneous fat space of the right palm had a slight blurring, which was considered a sign of inflammatory changes (Figure 7).
We tested the patient’s plasma for AIGAs due to the presence of the skin, lung, and intracranial multi-system lesions. Its titer was higher. The optical density (OD) value was still > 0.5 after a 2500-time dilution (OD normal range < 0.5).
AIGAs with intracranial infection and Sweet’s syndrome was the final diagnosis.
Ceftriaxone (1000 mg bid), amikacin (600 mg qd), azithromycin (500 mg b.i.d.), rifampicin (600 mg q.d.), ethambutol (1000 mg q.d.), and fluconazole (200 mg qd).
The patient had no fever, no obvious cough, no rash on the hand, and no headache after spending 20 d in the hospital before being discharged with medicine. The patient could get out of bed and care for herself because she was conscious. When she was seen again at the hospital two months later, all the signs were better than they had been. The patient continued receiving anti-mycobacterium therapy during future outpatient visits.
When a patient has multiple organ infections, typically from opportunistic infections without known immunosuppression, the diagnosis of adult-onset immunodeficiency syndrome associated with AIGAs should be considered. Since tests for established immunodeficiencies like human immunodeficiency virus are negative and antibodies to IFN-γ are strongly positive in this case, we gave the diagnosis of AIGAs some thought. Opportunistic infections, most frequently NTM infections and others, are linked to AIGAs[1-7]. Adult-onset immunodeficiency and NTM infection also showed a favorable additive interaction trend. We emphasize that even when culture results are negative, clinicians should be on the lookout for NTM infection in patients with AIGAs. Anti-IFN-γ was increased despite the lack of a confirmed NTM infection, and empirical anti-NTM treatment was successful in this case.
AIGAs damage multiple organs[10]. The lymphadenopathy, lungs, skin, and brain are the primary organs affected by this situation. CSF and brain MRI findings in this case led to the hypothesis of intracranial infection. Breakdown of the blood-brain barrier (BBB) is a common feature of many diseases of the central nervous system (CNS), including encephalitis. When the BBB is compromised in CNS diseases, there is a reduction in the transport of nutrients/oxygen, a quick influx of immune cells, and brain swelling that can exacerbate brain damage. According to a report, IFN-γ causes BBB leakage. In brain endothelial cells exposed to disease tissue lysate, IFN-γ is a barrier disruptor[14]. Through Rho kinase-mediated cytoskeletal contractions, IFN-γ reduces barrier properties in cultured brain endothelial cells, leading to junctional instability and cell-cell separations. We decided to use a medication that may penetrate the BBB to treat the intracranial infection present in this case.
In this instance, there was also skin damage. Skin pathologic or dermatologic examination findings supported the existence of Sweet’s syndrome. Neutrophilic dermatoses with negative microbial cultures were found by histopathology. Sweet’s syndrome is associated with adult-onset immunodeficiency, which includes lymphadenopathy, pustular lesions, and leukocytosis[15]. Clinicians should be aware of the possibility of an underlying adult-onset immunodeficiency since patients with Sweet’s syndrome have a specific clinical presentation that includes lymphadenopathy, pustular lesions, and leukocytosis.
In adults with severe and recurrent infections of multiple organs without other recognized risk factors, AIGAs should be considered. In AIGAs syndrome, the BBB may break down, leading to an intracranial infection. Sweet’s syndrome usually coexists with this syndrome.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kenzaka T, Japan; Oley MH, Indonesia S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Tang BS, Chan JF, Chen M, Tsang OT, Mok MY, Lai RW, Lee R, Que TL, Tse H, Li IW, To KK, Cheng VC, Chan EY, Zheng B, Yuen KY. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol. 2010;17:1132-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Lee WI, Huang JL, Wu TS, Lee MH, Chen IJ, Yu KH, Liu CY, Yang CH, Hsieh MY, Lin YL, Shih YF, Jaing TH, Huang SC, Kuo TT, Ku CL. Patients with inhibitory and neutralizing auto-antibodies to interferon-γ resemble the sporadic adult-onset phenotype of Mendelian Susceptibility to Mycobacterial Disease (MSMD) lacking Bacille Calmette-Guerin (BCG)-induced diseases. Immunobiology. 2013;218:762-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, Hsu AP, Patel SY, Olivier KN, Lulitanond V, Mootsikapun P, Anunnatsiri S, Angkasekwinai N, Sathapatayavongs B, Hsueh PR, Shieh CC, Brown MR, Thongnoppakhun W, Claypool R, Sampaio EP, Thepthai C, Waywa D, Dacombe C, Reizes Y, Zelazny AM, Saleeb P, Rosen LB, Mo A, Iadarola M, Holland SM. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 4. | Tanaka Y, Hori T, Ito K, Fujita T, Ishikawa T, Uchiyama T. Disseminated Mycobacterium avium complex infection in a patient with autoantibody to interferon-gamma. Intern Med. 2007;46:1005-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Koya T, Tsubata C, Kagamu H, Koyama K, Hayashi M, Kuwabara K, Itoh T, Tanabe Y, Takada T, Gejyo F. Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother. 2009;15:118-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, Martyak LA, Kubak B, Holland SM. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175:4769-4776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | O'Connell E, Rosen LB, LaRue RW, Fabre V, Melia MT, Auwaerter PG, Holland SM, Browne SK. The first US domestic report of disseminated Mycobacterium avium complex and anti-interferon-γ autoantibodies. J Clin Immunol. 2014;34:928-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS One. 2013;8:e76371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Hong GH, Ortega-Villa AM, Hunsberger S, Chetchotisakd P, Anunnatsiri S, Mootsikapun P, Rosen LB, Zerbe CS, Holland SM. Natural History and Evolution of Anti-Interferon-γ Autoantibody-Associated Immunodeficiency Syndrome in Thailand and the United States. Clin Infect Dis. 2020;71:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Aoki A, Sakagami T, Yoshizawa K, Shima K, Toyama M, Tanabe Y, Moro H, Aoki N, Watanabe S, Koya T, Hasegawa T, Morimoto K, Kurashima A, Hoshino Y, Trapnell BC, Kikuchi T. Clinical Significance of Interferon-γ Neutralizing Autoantibodies Against Disseminated Nontuberculous Mycobacterial Disease. Clin Infect Dis. 2018;66:1239-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Chetchotisakd P, Kiertiburanakul S, Mootsikapun P, Assanasen S, Chaiwarith R, Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis. 2007;45:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Phoompoung P, Ankasekwinai N, Pithukpakorn M, Foongladda S, Umrod P, Suktitipat B, Mahasirimongkol S, Kiertiburanakul S, Suputtamongkol Y. Factors associated with acquired Anti IFN- γ autoantibody in patients with nontuberculous mycobacterial infection. PLoS One. 2017;12:e0176342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Su SS, Zhang SN, Ye JR, Xu LN, Lin PC, Xu HY, Wu Q, Li YP. Disseminated Talaromyces marneffei And Mycobacterium avium Infection Accompanied Sweet's Syndrome In A Patient With Anti-Interferon-γ Autoantibodies: A Case Report. Infect Drug Resist. 2019;12:3189-3195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Bonney S, Seitz S, Ryan CA, Jones KL, Clarke P, Tyler KL, Siegenthaler JA. Gamma Interferon Alters Junctional Integrity via Rho Kinase, Resulting in Blood-Brain Barrier Leakage in Experimental Viral Encephalitis. mBio. 2019;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Kiratikanon S, Phinyo P, Rujiwetpongstorn R, Patumanond J, Tungphaisal V, Mahanupab P, Chaiwarith R, Tovanabutra N, Chiewchanvit S, Chuamanochan M. Adult-onset immunodeficiency due to anti-interferon-gamma autoantibody-associated Sweet syndrome: A distinctive entity. J Dermatol. 2022;49:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |