Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6949
Peer-review started: July 29, 2023
First decision: August 10, 2023
Revised: August 21, 2023
Accepted: September 11, 2023
Article in press: September 11, 2023
Published online: October 6, 2023
Malignant proliferating trichilemmal tumor (MPTT) is an infrequent malignant neoplasm originating from cutaneous appendages, with only a handful of documented cases. This report delineates a unique instance of MPTT situated in the neck, accompanied by lymph node metastasis. A comprehensive exposition of its clinical trajectory and imaging manifestation is presented, aiming to enhance comprehension and management of this atypical ailment.
Patient concerns: A 79-year-old male presented with a longstanding right neck mass persisting for over six decades, exhibiting recent enlargement over the past year. Diagnoses: Enhanced magnetic resonance imaging of the neck unveiled an elliptical mass on the right neck side, characterized by an ill-defined border and a heterogeneous signal pattern. The mass exhibited subdued signal intensity on T1-weighted imaging (T1WI) and a heterogeneous high signal on T2-weighted imaging (T2WI), interspersed with a lengthy T1 and T2 cystic signal motif. Close anatomical association with the submandibular gland joint was noted, and intravenous gadolinium diethylene triamine pentaacetic acid administration facilitated conspicuous enhancement. Substantial enhancement of the solid segment prompted an initial preoperative diagnosis of malignant nerve sheath tumor. However, post-surgery histopathological and immunohistochemical analysis conclusively confirmed the diagnosis as malignant hyperplastic external hair root sheath tumor. Intervention: Complete excision of the tumor was successfully executed. Outcomes: The patient experienced a favorable posto
Malignant proliferative trichilemmal tumor external hair root sheath tumor is a cystic-solid lesion, appearing as low signal on T1WI images or high signal on T2WI with enhancement of the solid component. Suspicions of malignancy are heightened when the tumor border is indistinct, tissue planes are breached, or when linear or patchy high signals are observed in the subcutaneous tissue on T1 liver acquisition with volume acceleration enhanced images along with intermediate signal on T2WI and restricted diffusion on diffusion-weighted imaging images. Strong consideration for malignancy should arise if there are signs of compromised adjacent tissue relationships or direct invasion evident on imaging. We have incorporated the above-mentioned content into the entire manuscript.
Core Tip: Malignant proliferating trichilemmal tumor (PTT) is an infrequent malignant neoplasm originating from cutaneous appendages, with only a handful of documented cases. This report delineates a unique instance of malignant PTT situated in the neck, accompanied by lymph node metastasis. A comprehensive exposition of its clinical trajectory and imaging manifestation is presented, aiming to enhance comprehension and management of this atypical ailment.
- Citation: Wang K, Wen JZ, Zhou SX, Ye LF, Fang C, Chen Y, Wang HX, Luo X. Malignant proliferative ependymoma of the neck with lymph node metastasis: A case report. World J Clin Cases 2023; 11(28): 6949-6954
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6949.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6949
Proliferating trichilemmal tumor (PTT) is an uncommon neoplasm originating from the outer hair sheath of the follicular isthmus within the dermis. It is characterized by well-defined borders, squamous cell attributes, and hyperkeratosis of the hair sheath[1]. The etiology of PTT remains enigmatic, although potential risk factors include infection, trauma, and exposure to ultraviolet light. The inaugural documented instance of PTT dates back to Jones’ report in 1966[2]. PTT predominantly emerges in sun-exposed regions of the body, with the head’s skin being the most frequent site[1,3]. Epidemiologically, this condition predominantly affects middle-aged and older women[4].
PTT represents a generally benign neoplasm with rare proclivity toward malignancy. In 1983, Saida et al[5] documented the initial case of malignant PTT (MPTT)[6]. Distorted mitosis, cytological diversity, peripheral infiltration, and aneuploidy have been observed across various cases[7]. PTT itself is a rarity, and the occurrence of MPTT accompanied by lymph node metastasis is exceedingly infrequent[8]. This article presents an exhaustive scrutiny of the clinical manifestation, imaging attributes, and surgical intervention strategy concerning a patient exhibiting typical clinical characteristics of malignant proliferative ependymoma in the neck, featuring lymph node metastasis.
Patient presents with a lump on the right side of the neck, which has been present for over 60 years and has increased in size over the past year.
The patient noticed the lump on the right side of the neck over 60 years ago, measuring approximately the size of a pigeon egg. At that time, there was no significant pain, limb numbness, voice hoarseness, chills, fever, productive cough, hemoptysis, abdominal distension, diarrhea, nausea, vomiting, chest discomfort, or dyspnea. Initial attention was not given to the lump. The neck lump has remained stable in size for over 60 years. However, during the last year, the patient observed a gradual enlargement of the lump on the right side of the neck, reaching the size of an egg.
The patient’s overall health has generally been good. There is no history of chronic conditions such as hypertension, diabetes, cardiovascular or cerebrovascular diseases, respiratory issues, digestive disorders, kidney diseases, infectious diseases, or endemic ailments. No surgical procedures or significant injuries have been experienced. Blood transfusions have not been undergone. The patient has reported allergies to cephalosporins and penicillin. Vaccination history is up-to-date according to local standards.
Personal history: The patient was born and raised in Quzhou, Zhejiang. Current residence is in Shiping village, Dazhou town. No history of residing in epidemic-prone areas. Education level is elementary school, and the patient's occupation is farming.
Family history: The cause of death for both parents remains unknown. There are one brother and one sister, both in good health, denying any shared illnesses or genetically linked conditions with the patient.
Vital signs: (1)Temperature 36.6 ℃, pulse 78 (beats/min), respiration 19 (breaths/min), blood pressure 122/80 mmHg, numeric rating scale pain score 0; (2) General: Height 155 cm, weight 54 kg, alert and oriented, normal development, well-nourished, autonomous posture, normal gait, no signs of illness, natural expressions, cooperative during examination; (3) Skin and mucosa: Normal color, no swelling, no rash, normal temperature and humidity, normal elasticity, no ecchymosis, normal hair distribution, no ulcers or scars, superficial lymph nodes not enlarged; (4) Head: Normal size and shape, no tenderness, no masses; (5) Eyes: No abnormalities in eyelids, eyelashes, conjunctiva, eyeballs, or sclera. Pupils are equal and react to light; (6) Ears: Hearing tested roughly with no abnormalities, no abnormalities in external ear, no secretions, no tenderness in mastoid; (7) Nose and throat: No abnormalities in nose, symmetrical nasal wing movement, adequate ventilation, no tenderness in nasal area, no tonsillar or pharyngeal abnormalities, trachea centrally located, no abnormalities in jugular veins; (8) Cardiovascular: No abnormal pulsations, heart sounds normal, no murmurs; (9) Lungs: Normal breathing movements, normal vocal resonance, no pleural friction rub, no subcutaneous crepitus, resonant percussion sound, lung borders at the 10th rib at the scapular line, respiration sounds clear, no pleural friction sounds; (10) Heart: Normal cardiac impulse, no abnormal pulsations, regular heart rate at 78 beats/min, no abnormal heart sounds, no pericardial friction rub; (11) Abdomen: Normal shape, abdominal breathing present, normal umbilicus, no scars, no abdominal wall tension, no tenderness, no rebound tenderness, no fluid wave, no masses, no McBurney’s point tenderness; (12) Liver: Liver not palpable below the rib, liver dullness and liver span normal, no tenderness, no pulsations, no hepatomegaly, no jugular venous reflux; (13) Gastrointestinal: Normal bowel sounds, no visible peristalsis, no abdominal masses; (14) Rectal examination: No abnormalities; (15) Genitalia: No abnormalities; (16) Spine and extremities: No abnormalities in spine, no tenderness, normal mobility, no abnormalities in limbs, no finger clubbing, no joint redness or swelling, no joint stiffness, no muscle wasting or tenderness, normal muscle strength, no varicose veins; and (17) Neurological: Normal cranial nerves, normal superficial and deep sensation, normal muscle strength, normal muscle tone, normal gait, normal reflexes.
Blood routine, liver and kidney function, electrolytes, coagulation function, and hepatitis B panel all show no significant abnormalities.
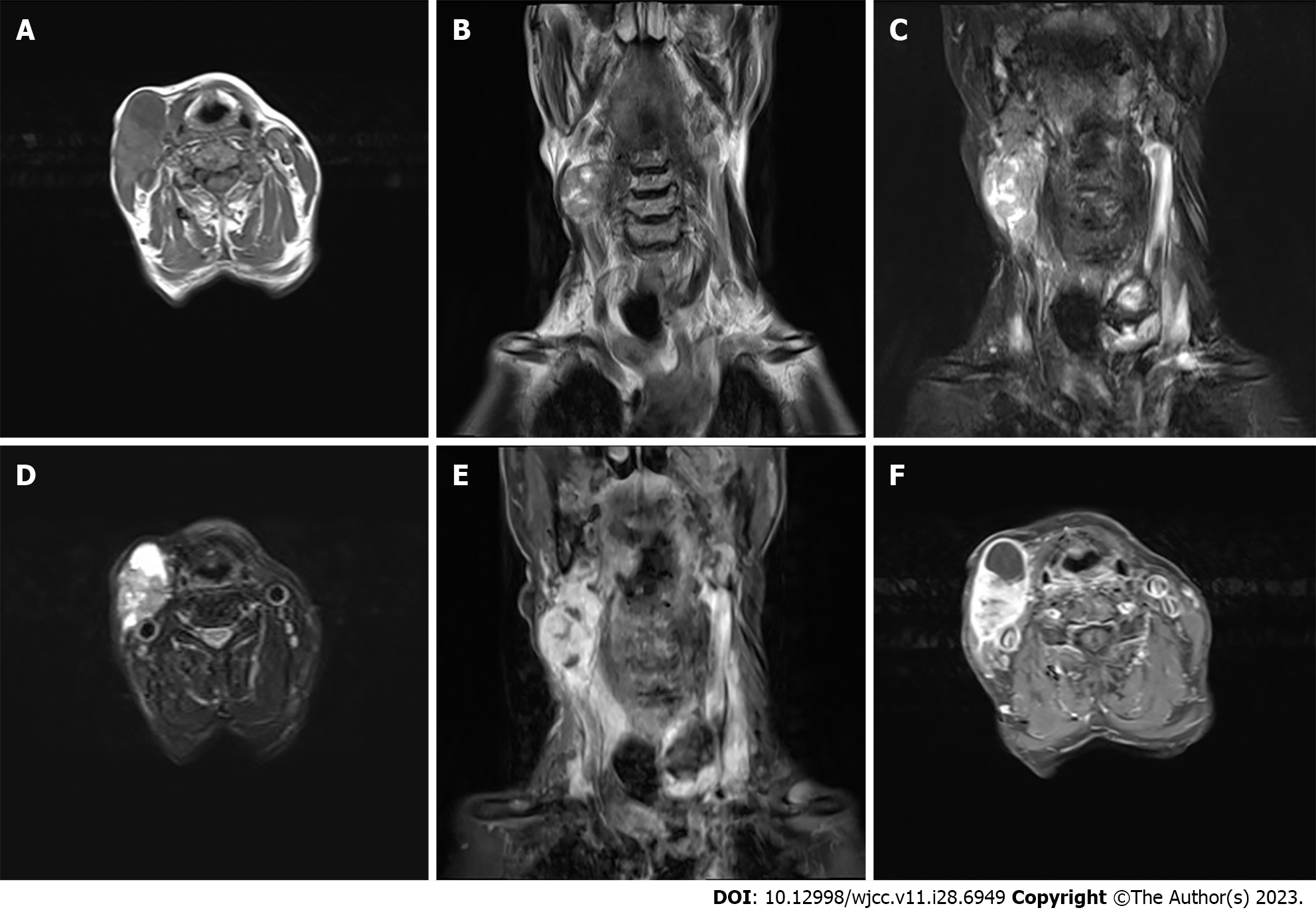
Enhanced magnetic resonance imaging of the neck unveiled an elliptical mass on the right neck side, characterized by an ill-defined border and a heterogeneous signal pattern. The mass exhibited subdued signal intensity on T1-weighted imaging (T1WI) and a heterogeneous high signal on T2-weighted imaging (T2WI) interspersed with a lengthy T1 and T2 cystic signal motif. Close anatomical association with the submandibular gland joint was noted, and intravenous gadolinium diethylene triamine pentaacetic acid administration facilitated conspicuous enhancement. Substantial enhancement of the solid segment prompted an initial preoperative diagnosis of malignant nerve sheath tumor, as shown in Figure 1. However, post-surgery histopathological and immunohistochemical analysis conclusively confirmed the diagnosis as malignant hyperplastic external hair root sheath tumor.
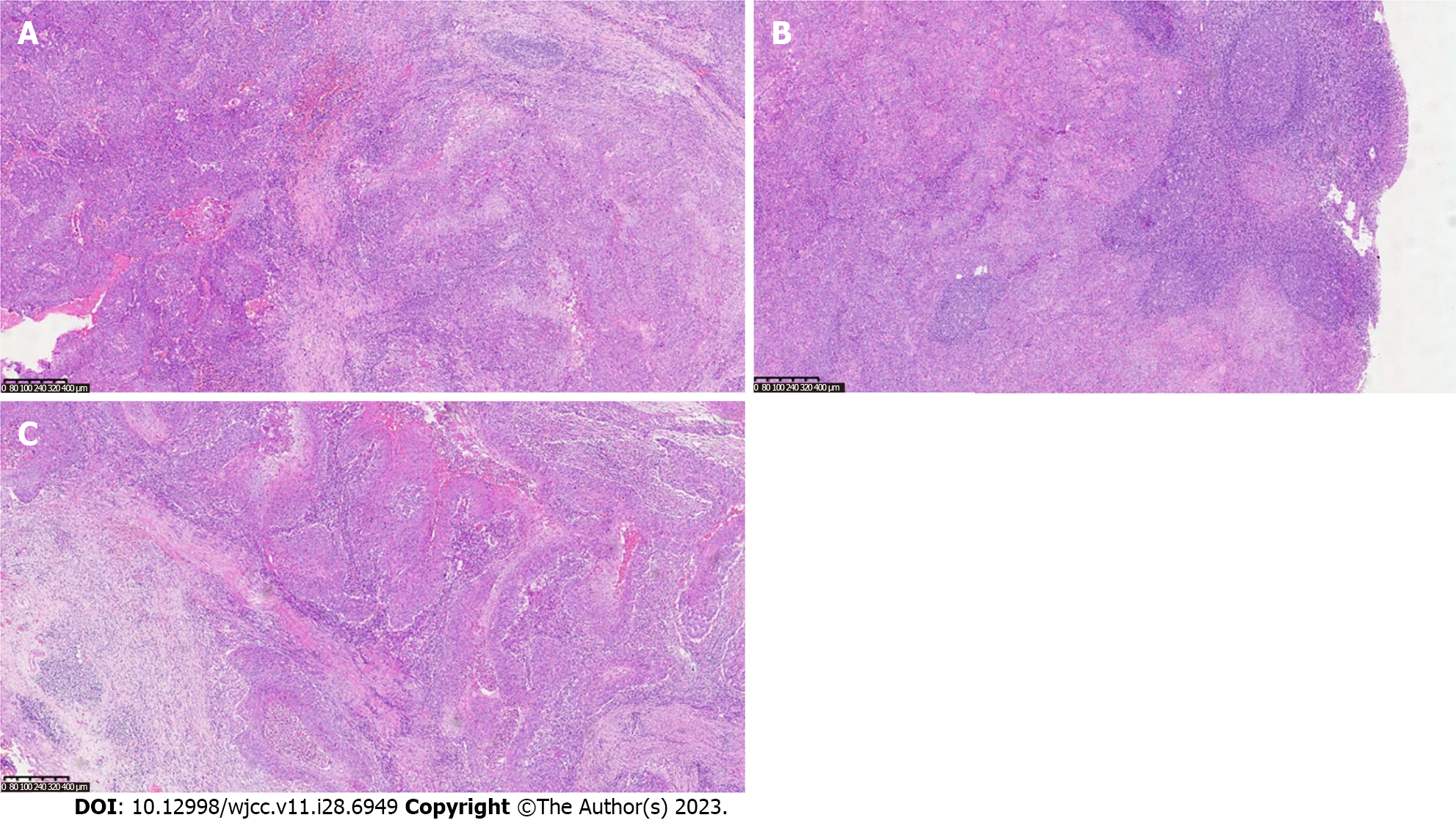
Our final diagnosis of this case was MPTT, and the results were confirmed pathologically, as shown in Figure 2.
Complete excision of the tumor was successfully executed.
The patient experienced a favorable postoperative recovery.
PTT is a rare cutaneous tumor, with research indicating that 80% of affected individuals are women over the age of 60[3]. Predominantly, 90% of these tumors manifest on the scalp, followed by occurrences on the forehead and neck skin[9]. Moreover, most instances of MPTT are conceived as localized malignancies evolving from pre-existing PTT lesions[10]. It is noteworthy that there exist limited reports of PTT undergoing malignant transformation on the scalp. Thus far, occurrences of MPTT with lymph node metastases within the neck’s skin are isolated and scarce. The radiographic representation of PTT/MPTT harmonizes with its clinical manifestation. Lesions on the head and neck typically exhibit characteristics of cystic-solid or cystic masses[10]. Appearing as low signal on T1WI images or high signal on T2WI with enhancement of the solid component. Suspicions of malignancy are heightened when the tumor border is indistinct, tissue planes are breached, or when linear or patchy high signals are observed in the subcutaneous tissue on T1 liver acquisition with volume acceleration enhanced images along with intermediate signal on T2WI and restricted diffusion on diffusion-weighted imaging images. Strong consideration for malignancy should arise if there are signs of compromised adjacent tissue relationships or direct invasion evident on imaging[11].
Proliferative ectodermal root sheath tumors are categorized into three groups: Group I (benign), marked by restricted lesions, mild nuclear heterogeneity, lack of mitotic activity, and absence of necrosis or lymphovascular infiltration; group II (low malignancy), characterized by histological irregularity, localized infiltration, and potential spread to dermal and subcutaneous tissues; and group III (highly malignant), characterized by infiltrative growth, pronounced nuclear heterogeneity, elevated mitotic and necrotic activity, and lymphatic and vascular infiltration[10,12]. Histologically, PTT masses typically display lobulated, well-defined contours without encapsulation. The tumor cells frequently resemble acanthocytes, forming irregular nests or cords, often exhibiting marked central keratinization. Small cystic or glandular lacunae containing keratinized material can be observed, resembling hair follicle differentiation. Nuclei present mild heterogeneity, occasionally with clusters of cells embedded in the mesenchyme and even beads of keratinization, a feature that can lead to confusion with squamous cell carcinoma. In contrast, MPTT tends to occupy deeper layers, mainly within the dermis or subcutaneous tissue. It displays infiltrative growth, notable nuclear heterogeneity, prominent atypical mitosis, and lymph node metastasis[13,14]. While no specific immunohistochemical markers exist for MPTT diagnosis, immunohistochemical studies continue to aid in distinguishing PTT from MPTT. In recent times, markers like p53 and Ki-67 have garnered attention, with certain studies revealing elevated expression of Ki-67 and p53 staining in MPTT. Concerning its biological behavior, PTT, especially MPTT, holds the potential for recurrence and metastasis, giving rise to local lymph node metastases or even distant systemic spread[9].
The tumor was situated within the right medial sternocleidomastoid muscle, exhibiting local infiltration, aberrant nuclear division, and lymphatic and vascular infiltration, categorically aligning with the highly malignant PTT category. In the context of the neck tumor, differentiation from lymphoma and malignant nerve sheath tumor is imperative. The primary intervention for MPTT entails surgical resection, necessitating extensive local excision of the tumor alongside approximately 1 cm of surrounding normal tissue to achieve a secure margin[4]. A stringent postoperative follow-up regimen is advisable for MPTT cases. Radiotherapy and chemotherapy have been applied for recurrent MPTT cases; however, given the rarity of this malignancy, published cases are insufficient for a comprehensive assessment of the efficacy and safety of these interventions. In summation, surgical excision remains the principal approach for MPTT management, with long-term monitoring imperative. Alternative therapeutic modalities warrant further comprehensive evaluation.
In summary, this case highlights the rarity and complexity of highly MPTT. Despite its infrequent occurrence, MPTT can lead to lymph node metastases in the neck’s skin. Radiographic and histological features correspond with clinical presentation, aiding diagnosis. Surgical resection remains the mainstay of treatment, while the efficacy of alternative therapies needs further exploration. Long-term follow-up is essential, emphasizing the need for continued research in MPTT management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Garg P, India; Jeyaraman M, India S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
| 1. | Zhou E, Xue X, Liu X, Zhang Y. Auricular Malignant Proliferating Trichilemmal Tumor: A Case Report. Ear Nose Throat J. 2022;1455613221127586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Jones EW. Proliferating epidermoid cysts. Arch Dermatol. 1966;94:11-19. [PubMed] [Cited in This Article: ] |
| 3. | Çoban K, Akkaya H, Aydın E. Proliferating Trichilemmal Tumor of the Auricula: A Very Rare Locus. Indian J Otolaryngol Head Neck Surg. 2019;71:1436-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Kemaloğlu CA, Öztürk M, Aydın B, Canöz Ö, Eğilmez O. Malignant proliferating trichilemmal tumor of the scalp: report of 4 cases and a short review of the literature. Case Reports Plast Surg Hand Surg. 2022;9:158-164. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Saida T, Oohara K, Hori Y, Tsuchiya S. Development of a malignant proliferating trichilemmal cyst in a patient with multiple trichilemmal cysts. Dermatologica. 1983;166:203-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Joshi TP, Marchand S, Tschen J. Malignant Proliferating Trichilemmal Tumor: A Subtle Presentation in an African American Woman and Review of Immunohistochemical Markers for This Rare Condition. Cureus. 2021;13:e17289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Azizi M, Ramezani M. Malignant proliferating trichilemmal tumor in abdominal wall: Report of a rare case at an uncommon site with literature review. Clin Case Rep. 2022;10:e05259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Alshaalan ZM, Patel P, Routt E, Ciocon D. Proliferating Pilar Tumor: Two Cases and a Review of the Literature. J Drugs Dermatol. 2021;20:1346-1348. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 9. | Wang G, Zhou X, Luo J, Hu Q, Zhang J. Case report: Malignant proliferating trichilemmal tumor of the thumb. Front Oncol. 2022;12:1005206. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 10. | Kawaguchi M, Kato H, Suzui N, Miyazaki T, Tomita H, Hara A, Matsuyama K, Seishima M, Matsuo M. Imaging findings of trichilemmal cyst and proliferating trichilemmal tumour. Neuroradiol J. 2021;34:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Singh P, Usman A, Motta L, Khan I. Malignant proliferating trichilemmal tumour. BMJ Case Rep. 2018;2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Weiffenbach A, Katz K, Rupley K, Carter A, Gottlieb A, Shulman K. Rapidly Enlarging Malignant Proliferating Trichilemmal Tumor in a Young Female. J Drugs Dermatol. 2018;17:1325-1327. [PubMed] [Cited in This Article: ] |
| 13. | Kearns-Turcotte S, Thériault M, Blouin MM. Malignant proliferating trichilemmal tumors arising in patients with multiple trichilemmal cysts: A case series. JAAD Case Rep. 2022;22:42-46. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 14. | Deshmukh BD, Kulkarni MP, Momin YA, Sulhyan KR. Malignant proliferating trichilemmal tumor: a case report and review of literature. J Cancer Res Ther. 2014;10:767-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |