Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4187
Peer-review started: April 12, 2023
First decision: April 26, 2023
Revised: May 4, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 16, 2023
This case report presents a patient with pyogenic spondylitis (PS) associated with lactation-related osteoporosis during pregnancy. The 34-year-old female patient experienced low back pain for one month, beginning one month postpartum, with no history of trauma or fever. Dual-energy X-ray absorptiometry of the lumbar spine revealed a Z-score of -2.45, leading to a diagnosis of pregnancy and lactation-associated osteoporosis (PLO). The patient was advised to cease breastfeeding and take oral calcium and active vitamin D. Despite these interventions, her symptoms worsened, and she had difficulty walking one week later, prompting her to revisit our hospital.
Lumbar magnetic resonance imaging (MRI) scans showed abnormal signals in the L4 and L5 vertebral bodies and intervertebral space, while an enhancement scan displayed abnormal enhanced high signals around the L4/5 intervertebral disc, suggesting a lumbar infection. A needle biopsy was performed for bacterial culture and pathological examination, culminating in a final diagnosis of pre
Both conditions primarily manifest as low back pain but require distinct treatments. In clinical practice, when diagnosing patients with pregnancy and lactation-associated osteoporosis, the possibility of spinal infection should be considered. A lumbar MRI should be conducted as needed to prevent delays in diagnosis and treatment.
Core Tip: This study described a special clinical condition of pregnancy and lactation-associated osteoporosis with pyogenic spondylitis. Both diseases present mainly as low back pain but are treated differently. The current study helps increase the awareness of this particular disease and reduce the clinical underdiagnosis rate.
- Citation: Zhai K, Wang L, Wu AF, Qian Y, Huang WM. Pregnancy and lactation-associated osteoporosis with pyogenic spondylitis: A case report. World J Clin Cases 2023; 11(17): 4187-4193
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4187.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4187
Pregnancy and lactation-associated osteoporosis (PLO) is a relatively rare clinical condition characterized by osteoporosis diagnosed during the third trimester of pregnancy up to 18 mo postpartum[1,2]. Its etiology remains unclear, but previous studies suggest that factors such as fetal osteogenesis during pregnancy, hormonal changes, genetic predisposition, and calcium loss after lactation may contribute to its development[3-5]. Common clinical manifestations include back or hip pain, and in severe cases, compressive vertebral fractures or hip fractures may occur[6,7].
Pyogenic spondylitis (PS) is a nonspecific bacterial infection affecting the vertebral body, intervertebral disc, or surrounding soft tissues. It predominantly occurs in the lumbar spine, involving both the intervertebral disc and adjacent vertebral bodies. In some instances, it can present as psoas abscess or spinal epidural abscess[8,9]. Although previous studies have documented cases of PS during pregnancy and breastfeeding[10,11], no reports have been found describing the co-occurrence of PLO and PS. When these conditions coexist, patients may initially experience only low back pain, which is easily overlooked, leading to delayed diagnosis and treatment and potentially irreversible consequences. This review presents a case of PLO with PS, which improved clinically after conservative treatment, in order to raise awareness of this particular disease and reduce the rate of clinical underdiagnosis.
A 34-year-old woman presented at our hospital with a one-month history of low back pain. She had undergone spontaneous delivery of a baby girl at a local hospital two months prior to this visit and had been breastfeeding the infant. The onset of her low back pain began one month after delivery and gradually worsened.
Upon her initial visit to our clinic, a dual-energy X-ray absorptiometry (DXA) was performed to assess her lumbar spine bone mineral density (BMD), revealing a Z-score of -2.45. Consequently, she was diagnosed with PLO and was advised to discontinue breastfeeding and to initiate oral calcium and vitamin D supplementation. However, her symptoms continued to worsen, leading to difficulty walking, and she was readmitted to the hospital one week later.
No trauma or fever was identified as a cause during the course of the disease. The patient had no history of chronic conditions, no exposure to cattle or sheep, no use of special drugs such as glucocorticoid.
The patient denied a family history of osteoporosis.
Upon readmission, the patient had a body temperature of 36.7 °C, height of 1.61 m, weight of 55 kg, and a body mass index (BMI) of 21.2 kg/m2. Physical examination revealed significantly limited range of lumbar motion and mild non-radiating pain upon percussion at the lumbar region. Muscle strength, sensation, and muscle tone in both lower limbs were found to be normal.
Laboratory tests were performed upon admission and the results are shown in Table 1. These tests included a complete blood count, sedimentation rate, C-reactive protein (CRP), calcitoninogen, and bone metabolism markers. Additionally, Brucella serum agglutination and tuberculosis antinuclear antibody tests were performed, with both yielding negative results. Serum calcium, phosphorus, and sex hormone levels are shown in Table 2.
| Project | CRP (mg/L) | ESR (mm/h) | WBC (109/L) | PCT (ng/mL) | 25-OH-D (ng/mL) | PINP (ng/L) | β-CTX | OST (ng/L) | PTH (pg/mL) |
| Reference values | 0-8 | 0-20 | 3.50-9.50 | 0-0.05 | 30-70 | 8.53-64.32 | 0.068-0.68 | 11-43 | 15-65 |
| This case | 8.43 | 25 | 4.98 | 0.04 | 12.96 | 62.74 | 1.430 | 22.21 | 12.0 |
| Project | AKP (U/L) | Ca (mmol/L) | P (mmol/L) | E2 (ng/L) | PROG (ug/L) | PRL (uIU/L) | LH (IU/L) | FSH (IU/L) |
| Reference values | 35-100 | 2.08-2.60 | 0.74-1.52 | 41-398 | 0.121-12.0 | 102-496 | 14-95.6 | 4.7-21.5 |
| This case | 69 | 2.39 | 1.51 | 5.33 | 0.052 | 938.7 | 3.20 | 4.02 |
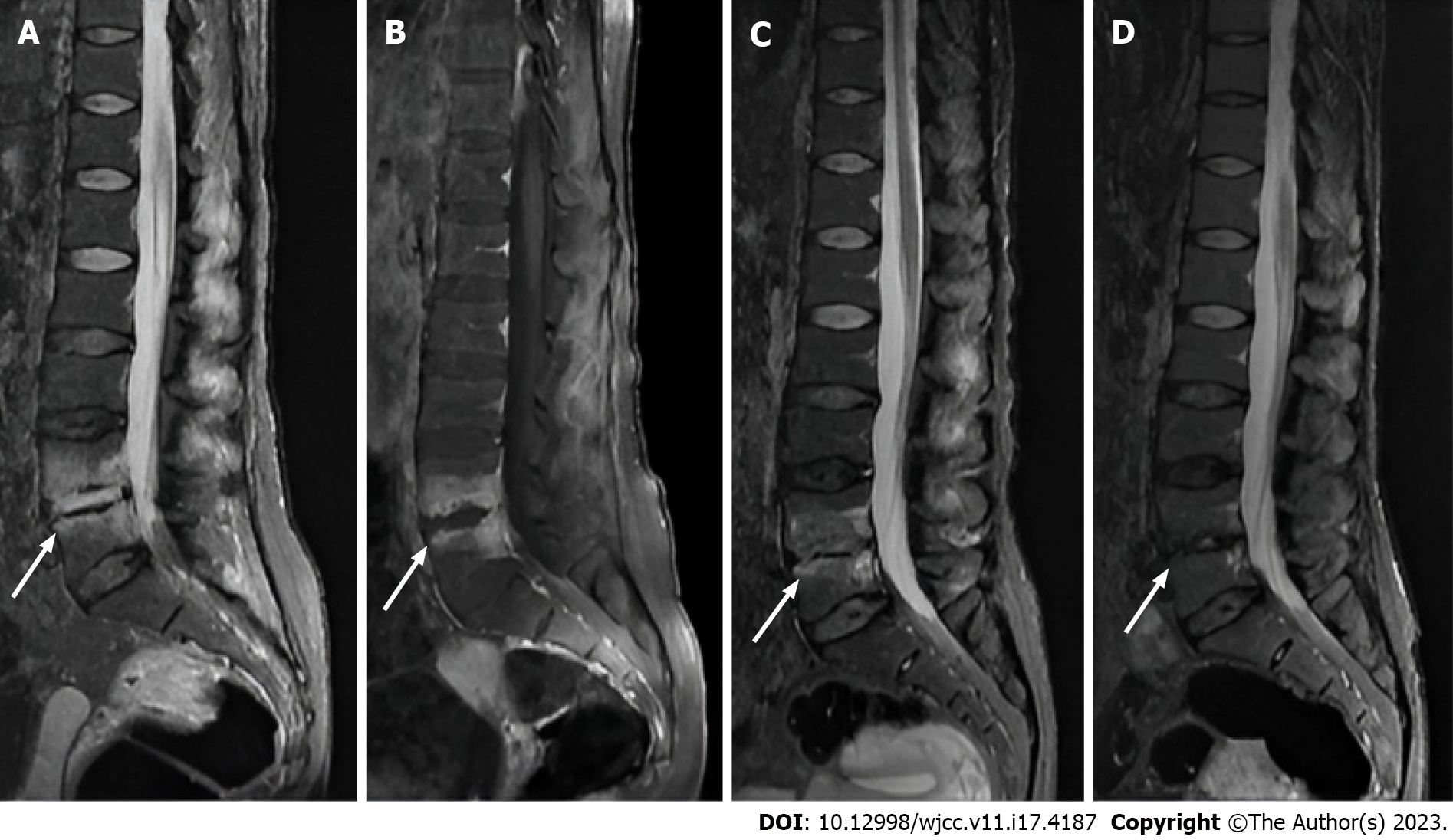
Magnetic resonance imaging (MRI) of the lumbar spine was performed upon admission, revealing abnormal signals in the L4 and L5 vertebral bodies and the intervertebral space. The contrast-enhanced scan demonstrated abnormally high signals surrounding the intervertebral space (Figure 1).
A comprehensive literature search was conducted using PubMed, Embase, and Web of Science as electronic databases. Citations were identified by employing a combination of the following keywords: “pregnancy and lactation-associated osteoporosis”, “spondylodiscitis”, “spinal infection”, “pyogenic spondylitis”, and “intervertebral space infection”, from the inception of each database through December 2022. The search terms encompassed the title, abstract, and topic terms. To date, no reports of concomitant PLO with PS have been identified.
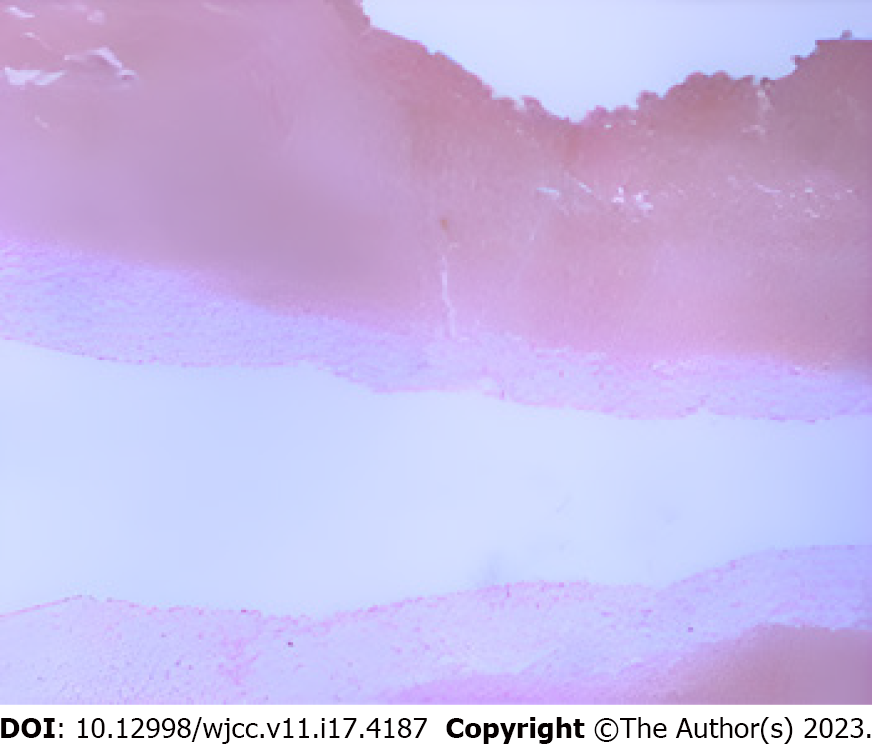
The patient was diagnosed with PLO and PS based on clinical assessment. To identify the causative bacteria, an X-ray-guided biopsy of the L5 vertebral body was performed. A cancellous bone strip was extracted from the L5 lesion using a puncture needle, with a portion sent for bacterial culture and drug sensitivity testing, and the remainder for pathological examination. The bacterial culture yielded negative results, while the pathological findings indicated lymphocyte, plasma cell, neutrophil, and eosinophil infiltration, consistent with acute suppurative inflammation (Figure 2).
The patient received anti-osteoporotic treatment comprising salmon calcitonin injections (50 u intramuscularly, once daily), calcium supplementation (600 mg orally, twice daily), and calcitriol capsules (0.5 ug orally, once daily) following admission. Due to the absence of positive bacterial culture results, empiric antibiotic therapy with cefuroxime sodium (1.5 g intravenous infusion, every 12 h) was initiated. The patient’s visual analog scale (VAS) pain score decreased from eight points at admission to three points after nine days of treatment during hospitalization.
The patient continued treatment with intravenous cefuroxime sodium until erythrocyte sedimentation rate (ESR) and CRP levels normalized. Upon discharge, the patient transitioned to oral cefuroxime sodium for approximately two weeks. Oral calcium and vitamin D supplementation were continued, while calcitonin was discontinued and substituted with oral menatetrenone soft capsules. One and five months post-discharge, the patient returned to the hospital for lumbar MRI scans (Figure 1). At the five-month follow-up, the patient exhibited a VAS score of zero for back pain and a Z-score of -1.94, as assessed by DXA of the lumbar spine, allowing for a return to normal life.
Previously, PLO was regarded as a rare disease; however, with increased awareness and recognition, the number of related studies has grown in recent years[2,12,13]. This study presents a case of PLO with PS, which is an uncommon clinical condition.
Currently, there is no consensus on PLO treatment, and clinical practice largely draws from postmenopausal osteoporosis management strategies[14]. Generally, patients with mild symptoms can cease breastfeeding and take oral calcium and vitamin D supplements. Those with severe symptoms or significant osteoporosis may be advised to incorporate anti-osteoporotic medications such as bisphosphonates, calcitonin, and teriparatide[15,16]. In this patient’s case, although infection was confirmed, the association between low back pain and osteoporosis remained unclear. Given the severity of the patient’s low back pain upon admission, calcitonin-based medications with superior analgesic properties were initially chosen. Bisphosphonates, commonly used for PLO treatment, were not selected due to concerns about their long-term deposition in bone and potential impact on the fetus in subsequent pregnancies[17], as the patient was in her first pregnancy and intended to conceive again in the future.
For patients diagnosed with PS, a pathogenic diagnosis should be obtained to inform the clinical use of antibiotics. In this case, the patient’s lack of fever or chills complicated the acquisition of bacteriological results from blood culture, prompting a puncture to collect tissue for pathogenic bacteria identification. Following the negative bacteriological findings, second-generation cephalosporin antibiotics were empirically chosen for treatment, given that Gram-positive bacteria are the most common pathogens in PS cases[18]. The selected antibiotics proved to be sensitive and effective, as evidenced by changes in ESR, CRP, and clinical symptoms after treatment.
In examining the relationship between pregnancy and PS, the literature has yet to identify pregnancy or lactation as risk factors for PS. Although some case reports have documented spinal tuberculosis[10,19] and PS[11] during pregnancy, a clear correlation between these conditions remains to be established. Pregnancy prompts physiological, hormonal, and immune system changes that create a unique, complex environment to protect both mother and fetus from pathogens. While there is no direct evidence indicating maternal immune system suppression during pregnancy, an increased risk of certain infections, such as those of the urinary and respiratory systems, has been observed[20,21]. Consequently, the incidence of potential PS during pregnancy might also be elevated.
The association between osteoporosis and PS has received limited attention in previous research. Bettag et al[22] found a high coexistence rate between osteoporosis and PS, which could be attributed to shared risk factors for both conditions. In their clinical practice, they noted a high incidence of internal fixation loosening following PS surgery. To determine whether this was related to osteoporosis, they conducted a retrospective analysis of 200 patients with PS who had undergone surgery. By assessing thoracolumbar spine computed tomography (CT) scans and measuring BMD using vertebral CT values, they discovered that 95 of the 200 patients (48%) exhibited preoperative osteoporosis. Although the underlying mechanism remains unclear, Bettag et al[22] emphasized that both osteoporosis and PS share risk factors such as smoking, oral glucocorticoid use, and malnutrition. Similarly, studies on PLO have demonstrated that smoking, glucocorticoid use, malnutrition, and low BMI are risk factors for PLO[12,14], suggesting a possible link between PLO and PS development. In addition, some underlying factors such as environment, metabolism, and diet factors may also contribute to osteoporosis in patients. Recent studies have shown that cadmium exposure and nonalcoholic fatty liver disease in adolescents are inversely correlated with BMD[23,24], while caffeine intake in children and adolescents is positively correlated with BMD[25]. However, further research is needed to confirm the effects of these factors specifically in patients with the PLO.
In summary, the concurrent presence of PLO and septic spondylitis can be easily overlooked, making early MRI examination crucial. As PLO and septic spondylitis share common risk factors, a potential correlation between the two conditions may exist. Future studies should investigate the specific coexisting incidence and the mechanisms underlying this association.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barbosa OA, Brazil; Liu M, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Kurabayashi T, Morikawa K. [Epidemiology and pathophysiology of post-pregnancy osteoporosis]. Clin Calcium. 2019;29:39-45. [PubMed] [Cited in This Article: ] |
| 2. | Hardcastle SA, Yahya F, Bhalla AK. Pregnancy-associated osteoporosis: a UK case series and literature review. Osteoporos Int. 2019;30:939-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Curtis EM, Parsons C, Maslin K, D'Angelo S, Moon RJ, Crozier SR, Gossiel F, Bishop NJ, Kennedy SH, Papageorghiou AT, Fraser R, Gandhi SV, Prentice A, Inskip HM, Godfrey KM, Schoenmakers I, Javaid MK, Eastell R, Cooper C, Harvey NC; MAVIDOS Trial Group. Bone turnover in pregnancy, measured by urinary CTX, is influenced by vitamin D supplementation and is associated with maternal bone health: findings from the Maternal Vitamin D Osteoporosis Study (MAVIDOS) trial. Am J Clin Nutr. 2021;114:1600-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Butscheidt S, Tsourdi E, Rolvien T, Delsmann A, Stürznickel J, Barvencik F, Jakob F, Hofbauer LC, Mundlos S, Kornak U, Seefried L, Oheim R. Relevant genetic variants are common in women with pregnancy and lactation-associated osteoporosis (PLO) and predispose to more severe clinical manifestations. Bone. 2021;147:115911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Leere JS, Vestergaard P. Calcium Metabolic Disorders in Pregnancy: Primary Hyperparathyroidism, Pregnancy-Induced Osteoporosis, and Vitamin D Deficiency in Pregnancy. Endocrinol Metab Clin North Am. 2019;48:643-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Raffaetà G, Mazzantini M, Menconi A, Bottai V, Falossi F, Celauro I, Guido G. Osteoporosis with vertebral fractures associated with pregnancy: two case reports. Clin Cases Miner Bone Metab. 2014;11:136-138. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Jun Jie Z, Ai G, Baojun W, Liang Z. Intertrochanteric fracture in pregnancy- and lactation-associated osteoporosis. J Int Med Res. 2020;48:300060519858013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Waheed G, Soliman MAR, Ali AM, Aly MH. Spontaneous spondylodiscitis: review, incidence, management, and clinical outcome in 44 patients. Neurosurg Focus. 2019;46:E10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Babic M, Simpfendorfer CS. Infections of the Spine. Infect Dis Clin North Am. 2017;31:279-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Nigam A, Prakash A, Pathak P, Abbey P. Bilateral psoas abscess during pregnancy presenting as an acute abdomen: atypical presentation. BMJ Case Rep. 2013;2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Bajwa ZH, Ho C, Grush A, Kleefield J, Warfield CA. Discitis associated with pregnancy and spinal anesthesia. Anesth Analg. 2002;94:415-416, table of contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Qian Y, Wang L, Yu L, Huang W. Pregnancy- and lactation-associated osteoporosis with vertebral fractures: a systematic review. BMC Musculoskelet Disord. 2021;22:926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Chaniotakis C, Koutserimpas C, Raptis K, Zafeiris E, Alpantaki K, Effraimidis G. Pregnancy associated osteoporotic vertebral fractures: an underdiagnosed condition of back pain. J Musculoskelet Neuronal Interact. 2021;21:332-334. [PubMed] [Cited in This Article: ] |
| 14. | Tuna F, Akleylek C, Özdemir H, Demirbağ Kabayel D. Risk factors, fractures, and management of pregnancy-associated osteoporosis: a retrospective study of 14 Turkish patients. Gynecol Endocrinol. 2020;36:238-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Lampropoulou-Adamidou K, Trovas G, Triantafyllopoulos IK, Yavropoulou MP, Anastasilakis AD, Anagnostis P, Toulis KA, Makris K, Gazi S, Balanika A, Tournis S. Teriparatide Treatment in Patients with Pregnancy- and Lactation-Associated Osteoporosis. Calcif Tissue Int. 2021;109:554-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Ijuin A, Yoshikata H, Asano R, Tsuburai T, Kikuchi R, Sakakibara H. Teriparatide and denosumab treatment for pregnancy and lactation-associated osteoporosis with multiple vertebral fractures: A case study. Taiwan J Obstet Gynecol. 2017;56:863-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Vujasinovic-Stupar N, Pejnovic N, Markovic L, Zlatanovic M. Pregnancy-associated spinal osteoporosis treated with bisphosphonates: long-term follow-up of maternal and infants outcome. Rheumatol Int. 2012;32:819-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Nakamura T, Morimoto T, Katsube K, Yamamori Y, Mashino J, Kikuchi K. Clinical characteristics of pyogenic spondylitis and psoas abscess at a tertiary care hospital: a retrospective cohort study. J Orthop Surg Res. 2018;13:302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Datta S, Spencer J. Cutaneous tuberculosis in pregnancy. J Obstet Gynaecol. 2004;24:455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal Immunological Adaptation During Normal Pregnancy. Front Immunol. 2020;11:575197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 21. | Fuhler GM. The immune system and microbiome in pregnancy. Best Pract Res Clin Gastroenterol. 2020;44-45:101671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Bettag C, Abboud T, von der Brelie C, Melich P, Rohde V, Schatlo B. Do we underdiagnose osteoporosis in patients with pyogenic spondylodiscitis? Neurosurg Focus. 2020;49:E16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Xie R, Liu Y, Wang J, Zhang C, Xiao M, Liu M, Zhang Y. Race and Gender Differences in the Associations Between Cadmium Exposure and Bone Mineral Density in US Adults. Biol Trace Elem Res. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 24. | Xie R, Zhang Y, Yan T, Huang X, Xie S, Liu C, Liu M. Relationship between nonalcoholic fatty liver disease and bone mineral density in adolescents. Medicine (Baltimore). 2022;101:e31164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Luo J, Liu M, Zheng Z, Zhang Y, Xie R. Association of urinary caffeine and caffeine metabolites with bone mineral density in children and adolescents. Medicine (Baltimore). 2022;101:e31984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |