Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2528
Peer-review started: December 21, 2022
First decision: January 20, 2023
Revised: February 1, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 16, 2023
Prostate cancer (PC) is currently the most common malignant tumor of the genitourinary system in men. Radical prostatectomy (RP) is recommended for the treatment of patients with localized PC. Adjuvant hormonal therapy (AHT) can be administered postoperatively in patients with high-risk or locally advanced PC. Chemotherapy is a vital remedy for castration-resistant prostate cancer (CRPC), and may also benefit patients with PC who have not progressed to CRPC.
A 68-year-old male was admitted to our hospital because of urinary irritation and dysuria with increased prostate-specific antigen (PSA) levels. After detailed examination, he was diagnosed with PC and treated with laparoscopic RP on August 3, 2020. AHT using androgen deprivation therapy (ADT) was performed postoperatively because of the positive surgical margin, extracapsular extension, and neural invasion but lasted only 6 mo. Unfortunately, he was diagnosed with rectal cancer about half a year after self-cessation of AHT, and was then treated with laparoscopic radical rectal resection and adjuvant chemotherapy using the capecitabine plus oxaliplatin (CapeOx) regimen. During the entire treatment process, the patient's PSA level first declined significantly after treatment of PC with laparoscopic RP and ADT, then rebounded because of self-cessation of ADT, and finally decreased again after CapeOx chemotherapy.
CapeOx chemotherapy can reduce PSA levels in patients with high-risk locally advanced PC, indicating that CapeOx may be an alternative chemotherapy regimen for PC.
Core Tip: Chemotherapy is mainly used for castration-resistant prostate cancer (PC) (CRPC) and may also benefit patients with PC who have not progressed to CRPC. We describe a patient with high-risk locally advanced PC treated with laparoscopic radical prostatectomy plus short-term adjuvant hormonal therapy and rectal cancer treated with laparoscopic radical rectal resection plus adjuvant chemotherapy, and found that prostate-specific antigen declined after the capecitabine plus oxaliplatin chemotherapy.
- Citation: Zou Q, Shen RL, Guo X, Tang CY. Prostate-specific antigen reduction after capecitabine plus oxaliplatin chemotherapy: A case report. World J Clin Cases 2023; 11(11): 2528-2534
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2528.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2528
Prostate cancer (PC) is the second most commonly diagnosed cancer in men, with 1414259 newly diagnosed cases worldwide in 2020[1]. The treatment of patients with PC differs according to the difference in the overall Gleason score and disease risk, and the main treatment methods include radical surgery (with or without dissection of enlarged lymph nodes), radical radiotherapy, hormone therapy, chemotherapy, and immunotherapy. Chemotherapy for PC is often used in combination with androgen deprivation therapy (ADT) for metastatic castration-resistant prostate cancer (CRPC). Taxanes such as docetaxel plus standard ADT prolong the median overall survival compared to ADT alone[2]. There is no clear conclusion regarding the advantages of chemotherapy after radical surgery for non-androgen-resistant PC, and clinical trials are underway[3].
Capecitabine plus oxaliplatin (CapeOx) is a commonly used chemotherapy regimen for stage III rectal cancer[4]. However, few studies have examined the effect of CapeOx on the recurrence rate in patients after radical prostatectomy (RP). We observed a patient with locally advanced PC treated with laparoscopic RP plus short-term ADT and metastatic rectal cancer treated with laparoscopic radical rectal resection (RRR) plus adjuvant chemotherapy using the CapeOx regimen, and found that the prostate-specific antigen (PSA) level declined after CapeOx chemotherapy in the absence of ADT. This report provides a new approach for the selection of chemotherapy regimens for patients with high-risk locally advanced PC after radical resection.
A 68-year-old male presented with urinary irritation and dysuria for one year and increased PSA levels for one month.
The patient was admitted to our hospital in July 2020 with urinary irritation and dysuria for 1 year, and his total serum PSA level was found to increase to 44.499 ng/mL in a health examination 1 mo prior to admission. The patient had no symptoms of urinary retention, hematuria, abdominal distension, or fever.
The patient had a history of hypertension for 10 years. He was treated with amlodipine besylate and irbesartan hydrochlorothiazide tablets, and his blood pressure was well controlled. Apart from hypertension, the patient had no other significant diseases.
The patient had no noticeable personal history and no similar family history.
Digital rectal examination (DRE) findings after admission were as follows: The prostate was II degree large, tough, with a shallow central sulcus, and no tenderness; induration was found in the left lobe of the prostate, with no obvious tenderness; the anal sphincter was not loose; there was no blood on the glove, and there were no palpable enlarged lymph nodes in the bilateral inguinal area.
Routine blood and urine tests, blood biochemistry, and coagulation function parameters were all normal, and detection of hepatitis B, hepatitis C, human immunodeficiency virus, and syphilis were all negative. However, the total serum PSA level further increased to 49.689 ng/mL after admission.
Ultrasound of the urinary system showed prostate hyperplasia with calcification (full shape of the prostate, approximately 50 mm in left-right diameter, 39 mm in upper-lower diameter, 40 mm in front-back diameter, thickened and uneven echo, imbalance in the ratio of internal and external glands, and multiple strong spots in the prostate). Nuclear magnetic resonance imaging revealed abnormal signals in the left and posterior parts of the peripheral zone of the prostate, suggesting the presence of PC. Therefore, ultrasound-guided needle biopsy was performed. Pathological examination of the left prostate puncture revealed an acinar adenocarcinoma with a Gleason score of 4 + 3 = 7, and examination of the right prostate puncture showed an acinar adenocarcinoma with a Gleason score of 3 + 4 = 7. Positron emission tomography-computed tomography revealed prostatic hyperplasia with calcification and nodular heterogeneous uptake of fluorodeoxyglucose in the peripheral zone.
According to the patient’s history, DRE findings, relevant imaging examinations, and needle biopsy results, a preoperative diagnosis of PC was confirmed. Postoperative pathology further determined the tumor-node-metastasis stage of PC.
The patient underwent laparoscopic RP on August 3, 2020. Postoperative pathological examination revealed prostatic acinar adenocarcinoma with a Gleason score of 4 + 3 = 7 and International Society of Urological Pathology grade of 3. The tumor-invasive areas included the bottom, body, and apex of the prostate. Involvement of the extracapsular extension and fibrous integument was also observed. The left and right seminal vesicles did not invade, and intravascular tumor embolus was not detected; however, neural invasion was observed. The left and right vas deferens were not affected, in contrast to the distal and proximal urethral margins. The patient was diagnosed with T3aN0M0 with a positive margin (R1) due to extracapsular extension and neural invasion. Therefore, adjuvant hormonal therapy (AHT) was requisite, and oral bicalutamide 50 mg per day plus subcutaneous leuprorelin 3.75 mg per 28 d (maximal androgen blockade) were used for ADT after surgery.
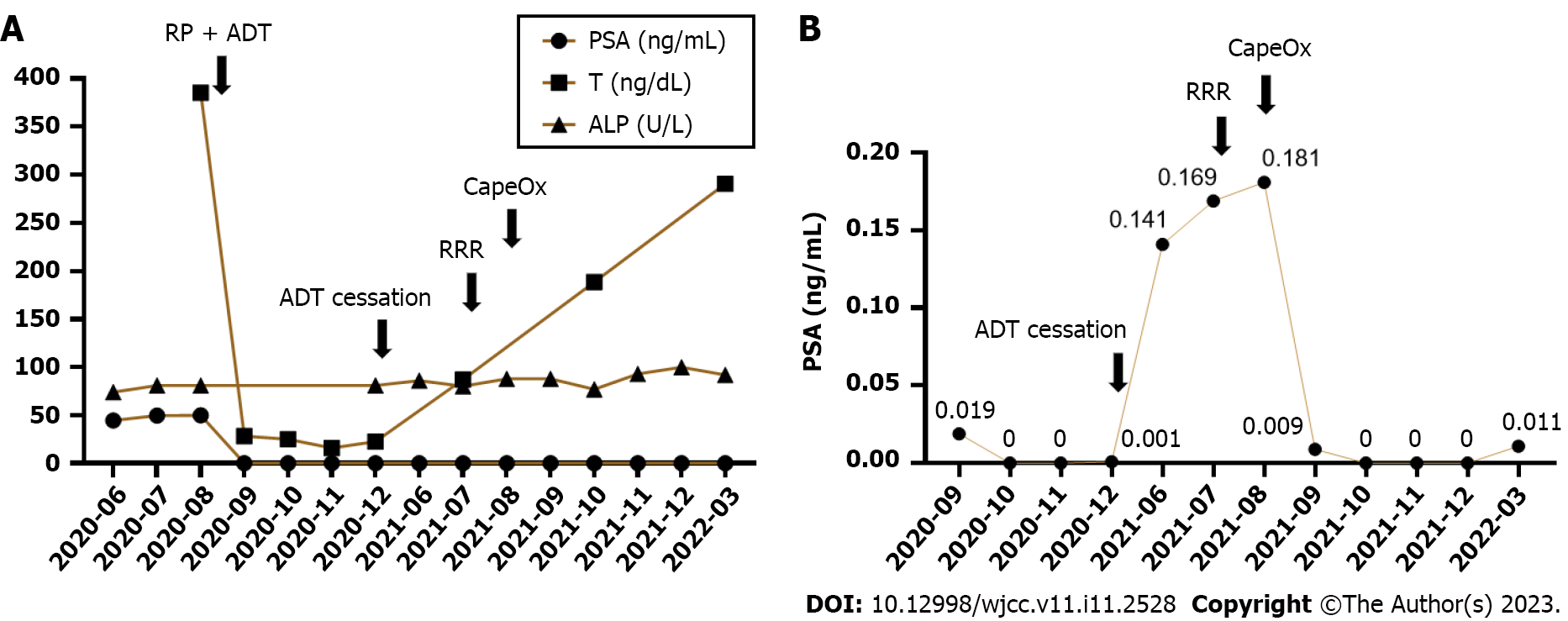
The patient was regularly treated until January 2021 for a total of 6 mo, and ADT was discontinued for personal reasons. In June 2021, the patient underwent an annual health examination, which unexpectedly revealed a rectal mass. Unfortunately, the patient was later diagnosed with rectal cancer via colonoscopic biopsy. Therefore, the patient was readmitted to the hospital for rectal cancer treatment, and laparoscopic RRR was performed in July 2021. Postoperative pathology revealed a protruding moderately differentiated adenocarcinoma (about 1.6 cm × 1.1 cm × 0.5 cm in size) with full-thickness invasion, accompanied by intravascular tumor embolus formation, and metastasis in 5 of the 7 resected mesenteric lymph nodes. The rectal cancer stage was pT3N2aM0, stage IIIB. Therefore, the CapeOx regimen was used for chemotherapy after the operation in August 2021, and the cycle lasted for half a year. At follow-up, we found that the patient’s PSA and testosterone levels both declined significantly after treatment of PC with laparoscopic RP and ADT in August 2020, but rebounded after the self-cessation of ADT in January 2021, while the alkaline phosphatase level remained stable (Figure 1). Surprisingly, the PSA level decreased again after CapeOx chemotherapy, eventually falling to zero (Figure 1B). However, a slight increase in PSA was observed in March 2022, the second month following the completion of chemotherapy (Figure 1B).
According to the EAU-EANM-ESTRO-ESUR-SIOG guidelines on PC, this patient was grouped into the high-risk group before surgery, and there is no unified standard regimen for the treatment of high-risk PC[5]. Either radical surgery or radiotherapy can be chosen, and RP is generally recommended for patients whose tumors do not invade the urethral sphincter or anchor the pelvic wall[5].
Postoperatively, the patient had a positive surgical margin (R1), extracapsular extension, and neural invasion (T3aN0M0). Therefore, the patient’s diagnosis should be defined as high-risk locally advanced PC, and postoperative pelvic radiotherapy could be selected in this case. Three major randomized controlled trials have addressed the issue of postoperative adjuvant radiotherapy in such patients[6-8]. The current conclusion is that for pT3pN0 patients with postoperative PSA level < 0.1 ng/mL, there is a high risk of local recurrence due to a positive surgical margin (the most important factor), extracapsular extension, and/or invasion of the seminal vesicles. At present, there are two options available to patients: (1) Adjuvant radiotherapy to the surgical area immediately after the recovery of urination function; and (2) during close clinical follow-up, starting salvage radiotherapy when PSA exceeds 0.1 ng/mL. However, urinary and gastrointestinal side effects are common during and after radiotherapy, as reported in the EORTC 22991 trial, with approximately 50% of the patients suffering from acute urogenital toxicity of grade 1, 20% of grade 2, and 2% of grade 3; meanwhile, approximately 30% of the patients reported acute gastrointestinal toxicity of grade 1, 10% of grade 2, and less than 1% of grade 3. Common toxic reactions include dysuria, frequent urination, urinary retention, hematuria, diarrhea, rectal bleeding, and proctitis[9]. After communicating with the patient, the second option was finally chosen, and adjuvant radiotherapy was not performed for the time being.
AHT after radical resection of high-risk or locally advanced PC can provide good survival benefits. For patients with a high recurrence risk, a long course of endocrine therapy (2-3 years) can be administered after surgery[10]. The patient was administered ADT postoperatively, after which his PSA and testosterone levels declined rapidly, eventually decreasing to zero for PSA and to less than 50 ng/dL for testosterone. However, after only 6 mo of normative AHT, the patient stopped treatment spontaneously, and both PSA and testosterone levels began to rebound. Subsequently, the patient was diagnosed with rectal cancer approximately half a year after self-cessation of AHT. Because of his condition, adjuvant chemotherapy using the CapeOx regimen was performed after laparoscopic RRR, showing that CapeOx chemotherapy could visibly reduce the PSA level. In other words, CapeOx chemotherapy may effectively control PC progression.
Chemotherapy is primarily used for CRPC. Androgen-targeted therapy prolongs survival in patients with metastatic CRPC; however, most men still face progressive disease and require additional treatment. Taxanes have been shown to improve survival in metastatic CRPC and are first-line chemotherapy drugs for the treatment of PC. Docetaxel is a taxane that can improve the response rates in patients with metastatic CRPC in terms of pain, serum PSA level, quality of life, and overall survival[11]. However, despite a good initial response and survival benefit, almost all patients eventually develop resistance, which is an important barrier to long-term survival[12]. In addition, available studies have shown that platinum-based chemotherapy drugs can benefit patients with CRPC. Dorff et al[13] performed re-chemotherapy with oxaliplatin and pemetrexed in men with CRPC that had progressed after one or two cycles of chemotherapy, including taxane, and showed that 47 men received a median of six cycles of treatment with an overall response rate of 30%, 64% of them had a decrease in PSA, and 74% of them had clinical disease control. Moreover, Lee et al[14] treated 33 CRPC patients who had failed docetaxel with intravenous gemcitabine and oxaliplatin combined with oral prednisolone, and showed a PSA response rate of 55%. These two clinical trials suggest that oxaliplatin as the core chemotherapy regimen can be used in patients with CRPC after the failure of taxane-based chemotherapy. In another study, eight of 14 androgen-independent PC patients (57%) treated with CapeOx after first-line docetaxel had a PSA response defined as a 50% reduction from baseline PSA levels. Our findings are consistent with those of this research. Hence, CapeOx chemotherapy may fill the gap in the treatment of CRPC after taxane treatment and become the second- and third-line chemotherapy for patients with CRPC[15].
It is generally believed that platinum drugs are first transported to tumor cells through copper transporter 1. After entering tumor cells, platinum complexes usually undergo the activated step of chloride ligand replacement through water molecules or other small molecules containing sulfhydryl groups. Compared with the extracellular matrix, intracellular chloride concentrations decrease significantly, which promotes the transformation of cation hydrate, and platinum-based chemotherapeutic drugs bind to DNA through the formation of intracellular and interstrand cross-links, changing the DNA structure and causing DNA damage. DNA damage can arrest the cell cycle and induce apoptosis in rapidly proliferating tumor cells[16]. In a mouse model of PC xenograft, compared with mice treated with anti-programmed cell death protein 1 (PD-1) antibody monotherapy alone, mice treated with oxaliplatin combined with anti-PD-1 antibody achieved the best survival rate[17]. In addition, two immunogenic markers, HMGB1 and calreticulin, expressed in oxaliplatin-treated PC cell lines PTEN-CaP8 and PNEC30, were significantly upregulated after 24 h of oxaliplatin treatment[17]. These results indicate that oxaliplatin could activate the immunophenotype of PC cells. Prostate fibroblasts promote the progression of PC by secreting factors that facilitate tumor growth and induce migration and invasion of PC cells. Angiogenin (ANG) can significantly stimulate the invasion of type I collagen in fibroblasts associated with PC[18]. ANG, a member of the secretory ribonuclease superfamily, is upregulated in PC and mediates ribosomal RNA (rRNA) transcription in androgen-stimulated PC cells. Upon androgen stimulation, ANG undergoes nuclear translocation in androgen-dependent PC cells where it binds to the ribosomal DNA promoter and stimulates rRNA transcription. ANG antagonists inhibit androgen-induced rRNA transcription and proliferation in androgen-dependent PC cells[19]. Surprisingly, using a comprehensive multi-technology approach of crystallography, spectroscopy, and spectral analysis, Marzo et al[20] demonstrated that oxaliplatin could effectively bind ANG to inhibit the proliferation and migration of the PC cell line PC-3. This finding provides another research direction for the treatment of PC with oxaliplatin.
Chemotherapy for PC is mainly used for CRPC, while there are few clinical studies on the use of chemotherapy in patients with common PC after RP. The anticancer mechanism of oxaliplatin mainly causes DNA damage, thereby stopping the cell division cycle. In PC, oxaliplatin has a unique role in immunology and angiogenesis, which may also provide new directions for future studies on the anticancer mechanism of oxaliplatin.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Marickar F, India; Sarier M, Turkey S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 45299] [Article Influence: 15099.7] [Reference Citation Analysis (47)] |
| 2. | Sonnenburg DW, Morgans AK. Emerging Therapies in Metastatic Prostate Cancer. Curr Oncol Rep. 2018;20:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Ahlgren GM, Flodgren P, Tammela TLJ, Kellokumpu-Lehtinen P, Borre M, Angelsen A, Iversen JR, Sverrisdottir A, Jonsson E, Sengelov L; Investigators of the Scandinavian Prostate Cancer Study Number 12. Docetaxel Versus Surveillance After Radical Prostatectomy for High-risk Prostate Cancer: Results from the Prospective Randomised, Open-label Phase 3 Scandinavian Prostate Cancer Group 12 Trial. Eur Urol. 2018;73:870-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Liu FQ, Cai SJ. [Adjuvant and perioperative neoadjuvant therapy for colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22:315-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, Grivas N, Grummet J, Henry AM, van der Kwast TH, Lam TB, Lardas M, Liew M, Mason MD, Moris L, Oprea-Lager DE, van der Poel HG, Rouvière O, Schoots IG, Tilki D, Wiegel T, Willemse PM, Cornford P. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79:243-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 606] [Cited by in F6Publishing: 1395] [Article Influence: 348.8] [Reference Citation Analysis (0)] |
| 6. | Hackman G, Taari K, Tammela TL, Matikainen M, Kouri M, Joensuu T, Luukkaala T, Salonen A, Isotalo T, Pétas A, Hendolin N, Boström PJ, Aaltomaa S, Lehtoranta K, Hellström P, Riikonen J, Korpela M, Minn H, Kellokumpu-Lehtinen PL, Pukkala E, Hemminki A; FinnProstate Group. Randomised Trial of Adjuvant Radiotherapy Following Radical Prostatectomy Versus Radical Prostatectomy Alone in Prostate Cancer Patients with Positive Margins or Extracapsular Extension. Eur Urol. 2019;76:586-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Kneebone A, Fraser-Browne C, Duchesne GM, Fisher R, Frydenberg M, Herschtal A, Williams SG, Brown C, Delprado W, Haworth A, Joseph DJ, Martin JM, Matthews JHL, Millar JL, Sidhom M, Spry N, Tang CI, Turner S, Wiltshire KL, Woo HH, Davis ID, Lim TS, Pearse M. Adjuvant radiotherapy vs early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020;21:1331-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 8. | Parker CC, Clarke NW, Cook AD, Kynaston HG, Petersen PM, Catton C, Cross W, Logue J, Parulekar W, Payne H, Persad R, Pickering H, Saad F, Anderson J, Bahl A, Bottomley D, Brasso K, Chahal R, Cooke PW, Eddy B, Gibbs S, Goh C, Gujral S, Heath C, Henderson A, Jaganathan R, Jakobsen H, James ND, Kanaga Sundaram S, Lees K, Lester J, Lindberg H, Money-Kyrle J, Morris S, O'Sullivan J, Ostler P, Owen L, Patel P, Pope A, Popert R, Raman R, Røder MA, Sayers I, Simms M, Wilson J, Zarkar A, Parmar MKB, Sydes MR. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet. 2020;396:1413-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 9. | Matzinger O, Duclos F, van den Bergh A, Carrie C, Villà S, Kitsios P, Poortmans P, Sundar S, van der Steen-Banasik EM, Gulyban A, Collette L, Bolla M; EORTC Radiation Oncology Group. Acute toxicity of curative radiotherapy for intermediate- and high-risk localised prostate cancer in the EORTC trial 22991. Eur J Cancer. 2009;45:2825-2834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Dong BJ, He DL, Li MZ, Li YH, Li ZH, Liu RR, Liu ZH, Sheng XN, Wang L, Wang XH. [Safety consensus on classical endocrine therapy for prostate cancer]. Xiandai Miniao Waike Zazhi. 2018;23:248-258. [DOI] [Cited in This Article: ] |
| 11. | Nader R, El Amm J, Aragon-Ching JB. Role of chemotherapy in prostate cancer. Asian J Androl. 2018;20:221-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Ruiz de Porras V, Font A, Aytes A. Chemotherapy in metastatic castration-resistant prostate cancer: Current scenario and future perspectives. Cancer Lett. 2021;523:162-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Dorff TB, Tsao-Wei DD, Groshen S, Boswell W, Goldkorn A, Xiong S, Quinn DI, Pinski JK. Efficacy of oxaliplatin plus pemetrexed in chemotherapy pretreated metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2013;11:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Lee JL, Ahn JH, Choi MK, Kim Y, Hong SW, Lee KH, Jeong IG, Song C, Hong BS, Hong JH, Ahn H. Gemcitabine-oxaliplatin plus prednisolone is active in patients with castration-resistant prostate cancer for whom docetaxel-based chemotherapy failed. Br J Cancer. 2014;110:2472-2478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Gasent Blesa JM, Giner Marco V, Giner-Bosch V, Cerezuela Fuentes P, Alberola Candel V. Phase II trial of oxaliplatin and capecitabine after progression to first-line chemotherapy in androgen-independent prostate cancer patients. Am J Clin Oncol. 2011;34:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Zhang C, Xu C, Gao X, Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022;12:2115-2132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 181] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 17. | Zhou J, Yang T, Liu L, Lu B. Chemotherapy oxaliplatin sensitizes prostate cancer to immune checkpoint blockade therapies via stimulating tumor immunogenicity. Mol Med Rep. 2017;16:2868-2874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Jones ML, Ewing CM, Isaacsa WB, Getzenberg RH. Prostate cancer-derived angiogenin stimulates the invasion of prostate fibroblasts. J Cell Mol Med. 2012;16:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Li S, Hu MG, Sun Y, Yoshioka N, Ibaragi S, Sheng J, Sun G, Kishimoto K, Hu GF. Angiogenin mediates androgen-stimulated prostate cancer growth and enables castration resistance. Mol Cancer Res. 2013;11:1203-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Marzo T, Ferraro G, Cucci LM, Pratesi A, Hansson Ö, Satriano C, Merlino A, La Mendola D. Oxaliplatin inhibits angiogenin proliferative and cell migration effects in prostate cancer cells. J Inorg Biochem. 2022;226:111657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |