Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6536
Peer-review started: September 13, 2021
First decision: January 10, 2022
Revised: November 12, 2021
Accepted: May 8, 2022
Article in press: May 8, 2022
Published online: July 6, 2022
Immune checkpoint inhibitors have revolutionized the treatment of patients with unresectable metastatic malignant melanoma. In addition to systemic side effects, several usually mild ocular adverse effects have been reported. We report a case of rarely reported vision-threatening bilateral panuveitis with serous retinal detachment, thickened choroid, and chorioretinal folds associated with dabrafenib and trametinib targeted therapy for B-Raf proto-oncogene serine/threonine kinase (BRAF) mutant metastatic cutaneous melanoma.
A 59-year-old female patient with metastatic melanoma treated with dabrafenib and trametinib presented with blurry vision and central scotoma lasting for 3 d in both eyes. Clinical examination and multimodal imaging revealed inflammatory cells in the anterior chamber, mild vitritis, bullous multiple serous retinal detachments, and chorioretinal folds in both eyes. Treatment with dabrafenib and trametinib was suspended, and the patient was treated with topical and intravenous corticosteroids followed by oral corticosteroid treatment with a tapering schedule. One and a half months after the disease onset, ocular morphological and functional improvement was noted. Due to the metastatic melanoma dissemination, BRAF/mitogen-activated protein kinase inhibitors were reintroduced and some mild ocular adverse effects reappeared, which later subsided after receiving oral corticosteroids.
Patients on combination therapy with dabrafenib and trametinib may rarely develop severe bilateral panuveitis with a good prognosis. Further studies have to establish potential usefulness of ophthalmological examination for asymptomatic patients. Furthermore, appropriate guidelines for managing panuveitis associated with dabrafenib and trametinib should be established.
Core Tip: The effectiveness of B-Raf proto-oncogene serine/threonine kinase (BRAF)/mitogen-activated protein kinase (MEK) inhibitor therapy in terms of prolonging survival has revolutionized the treatment of metastatic melanoma and other cancers. Consequently, widespread use in several metastatic cancers could be anticipated in the future. Therefore, all possible adverse effects should be described. We report a case of very rare and severe bilateral panuveitis with transient visual loss associated with treatment of metastatic malignant melanoma. The purpose of our case report is to raise awareness of possible severe bilateral panuveitis associated with immune checkpoint inhibitor therapy and to show that ocular symptoms subside with BRAF/MEK inhibitor discontinuation and/or systemic corticosteroids. Further studies should establish guidelines for panuveitis management and evaluate the importance of ophthalmological examination for asymptomatic patients.
- Citation: Kiraly P, Groznik AL, Valentinčič NV, Mekjavić PJ, Urbančič M, Ocvirk J, Mesti T. Choroidal thickening with serous retinal detachment in BRAF/MEK inhibitor-induced uveitis: A case report. World J Clin Cases 2022; 10(19): 6536-6542
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6536.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6536
Targeted therapy drugs including B-Raf proto-oncogene serine/threonine kinase (BRAF) and mitogen-activated protein kinase (MEK) inhibitors have markedly improved the progression-free survival rate in unresectable BRAF mutant metastatic melanoma[1]. Both drugs inhibit the mitogen-activated protein kinase (MAPK) pathway and are commonly used in combination because BRAF inhibitor monotherapy resistance usually develops in 6-8 mo[2]. MEK inhibitors in combination help to overcome the described resistance, which further prolongs the overall survival[3]. Although benefits on overall survival rate are unequivocal, several adverse effects have been reported[4]. In patients on MEK inhibitor treatment, ocular adverse effects are very common, with an incidence of up to 90%. Ocular side effects range from blurred vision, periorbital oedema, and dry eyes to retinal toxicities such as MEK-associated retinopathy, retinal vein occlusion, and panuveitis[5]. A study reported ocular adverse effects in 22% of 568 patients treated with the BRAF inhibitor vemurafenib. The most common side effects were uveitis (4%), conjunctivitis (2.8%), and dry eyes (2.0%)[6]. A small case series of seven patients on vemurafenib treatment for metastatic melanoma reported bilateral non-granulomatous anterior uveitis as a side effect that responded well to local corticosteroid therapy, justifying continuation of vemurafenib treatment. In one patient, explosive bilateral panuveitis with hand movements visual acuity was described[7]. Although there are some published studies investigating ocular adverse effects with BRAF or MEK inhibitor monotherapy, there are only few case reports describing ocular side effect associated with the combination therapy with dabrafenib and trametinib[8-10]. Albertini et al[8] published a case report in which dabrafenib and trametinib therapy was associated with multifocal choroiditis reactivation and secondary choroidal neovascularization, which were successfully treated with ranibizumab. Another case report with the same combination therapy was published, reporting a patient with bilateral non-granulomatous panuveitis, pachychoroid, chorioretinal folds, and multiple foci of subretinal fluid accumulation. In this study, dabrafenib discontinuation and local corticosteroid treatment resulted in improved visual acuity and resolved serous neurosensory detachments[9]. Another article reporting a patient on dabrafenib and trametinib therapy described bilateral intermediate uveitis with late optic disc fluorescein leakage on angiography and 6/6 visual acuity[10].
A 59-year-old female patient presented to the University Eye Hospital complaining of vision worsening.
At presentation, the patient reported bilateral central scotoma and vision loss for about 3 d.
From childhood, the patient has had recurrent herpetic keratouveitis episodes in the right eye. From 2014, she has been treated at the Oncology Institute Ljubljana for skin melanoma. In 2014, she underwent surgical removal of skin melanoma in the abdominal area (Clark IV, Breslow 4mm, 5 mitoses/mm2) with no ulceration, one positive sentinel node in the left armpit, and 20 axillar lymph nodes with no metastasis. She had been treated with adjuvant high dose interferon alpha for 1 year. Afterwards, between 2017 and 2019, she underwent four melanoma metastases metastasectomies (R0) in different parts of the left breast, and radiotherapy (50 Gy) after the last metastasectomy. In July 2019, a fifth relapse (9 mm lesion) in the left breast was assessed by Positron emission tomography-computed tomography (PET-CT) and cytologically confirmed. After the patient refusing a mastectomy, systemic treatment with the checkpoint PD1 inhibitor pembrolizumab was initiated as PCR analysis confirmed BRAF V600E mutation. The treatment was discontinued after the fourth course, as she developed immune related pneumonitis that was successfully treated with oral corticosteroids. In January 2020, progression with new subcutaneous melanoma metastases under the left breast occurred and treatment with BRAF/MEK inhibitors (dabrafenib and trametinib) was prescribed. After the second course of treatment, PET-CT showed a complete response with metastases regression. At the time of presentation to the eye hospital, in August 2020, she was taking BRAF/MEK inhibitors for metastatic malignant melanoma (dabrafenib 150 mg twice daily and trametinib 2 mg once daily) with no side effects.
Apart from the aforementioned past illnesses, the patient’s personal and family history was unremarkable.
At presentation, the patient’s best corrected visual acuity was 0.5 in both eyes measured using the Snellen chart, and her intraocular pressure was 21 mmHg in the right eye (RE) and 18 mmHg in the left eye (LE). She had corneal scarring on her RE, and non-granulomatous endothelial precipitates, anterior chamber cells 2+, and vitritis with cells 2+ in both eyes. Fundus examination revealed multiple serous exudative neurosensory detachment around the optic nerve discs, vascular arcades, and macula in both eyes.
Blood tests excluded infectious uveitis causes [Bartonella sp., Borrelia sp., Treponema pallidum, Toxoplasma gondii, QuantiFERON-TB Gold (QFT)]. Only Toxoplasma gondii IgG antibodies were positive (66.40 IU/mL). Serum testing, aqueous, and vitreous humor samples to exclude herpetic infection were not obtained.
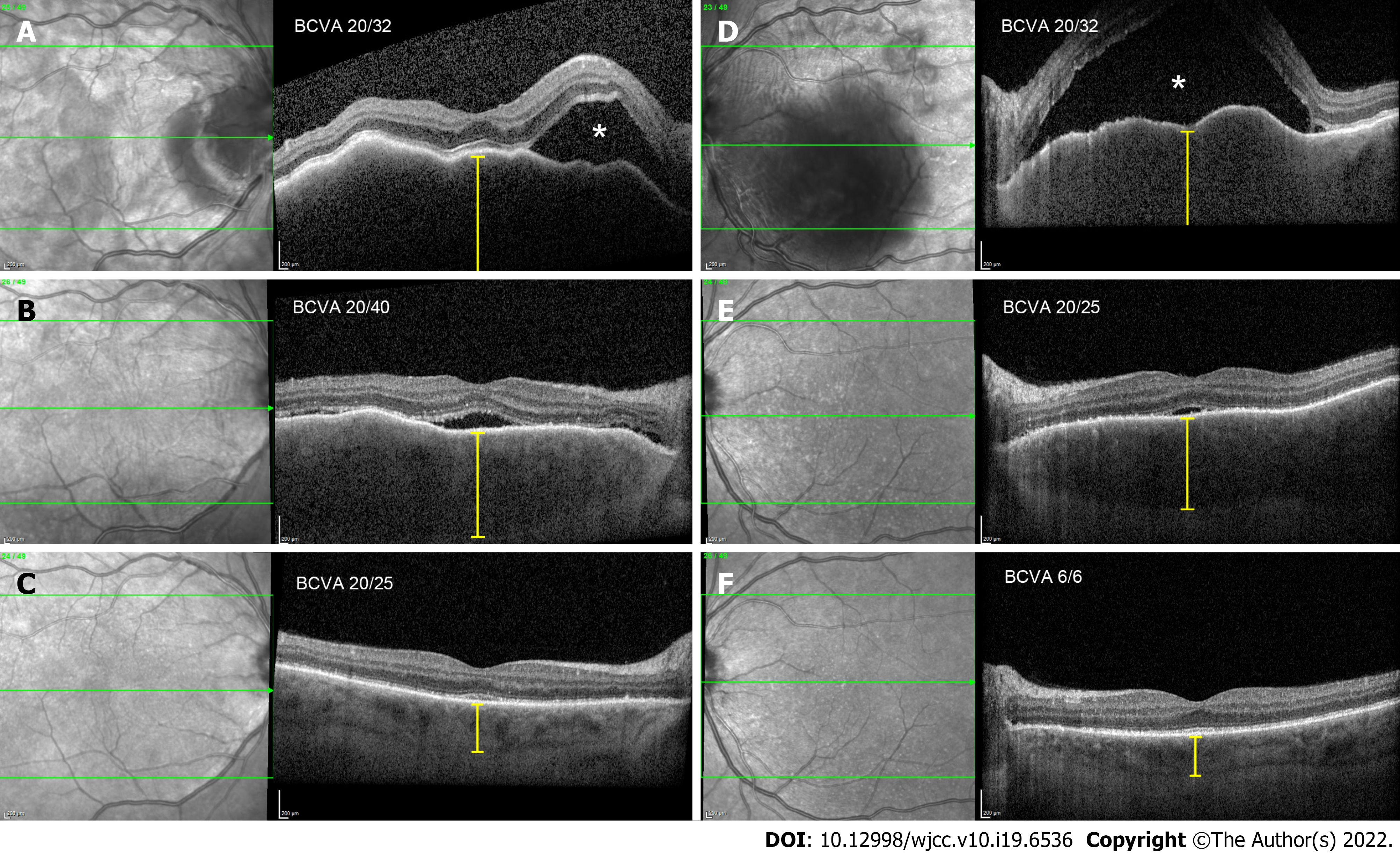
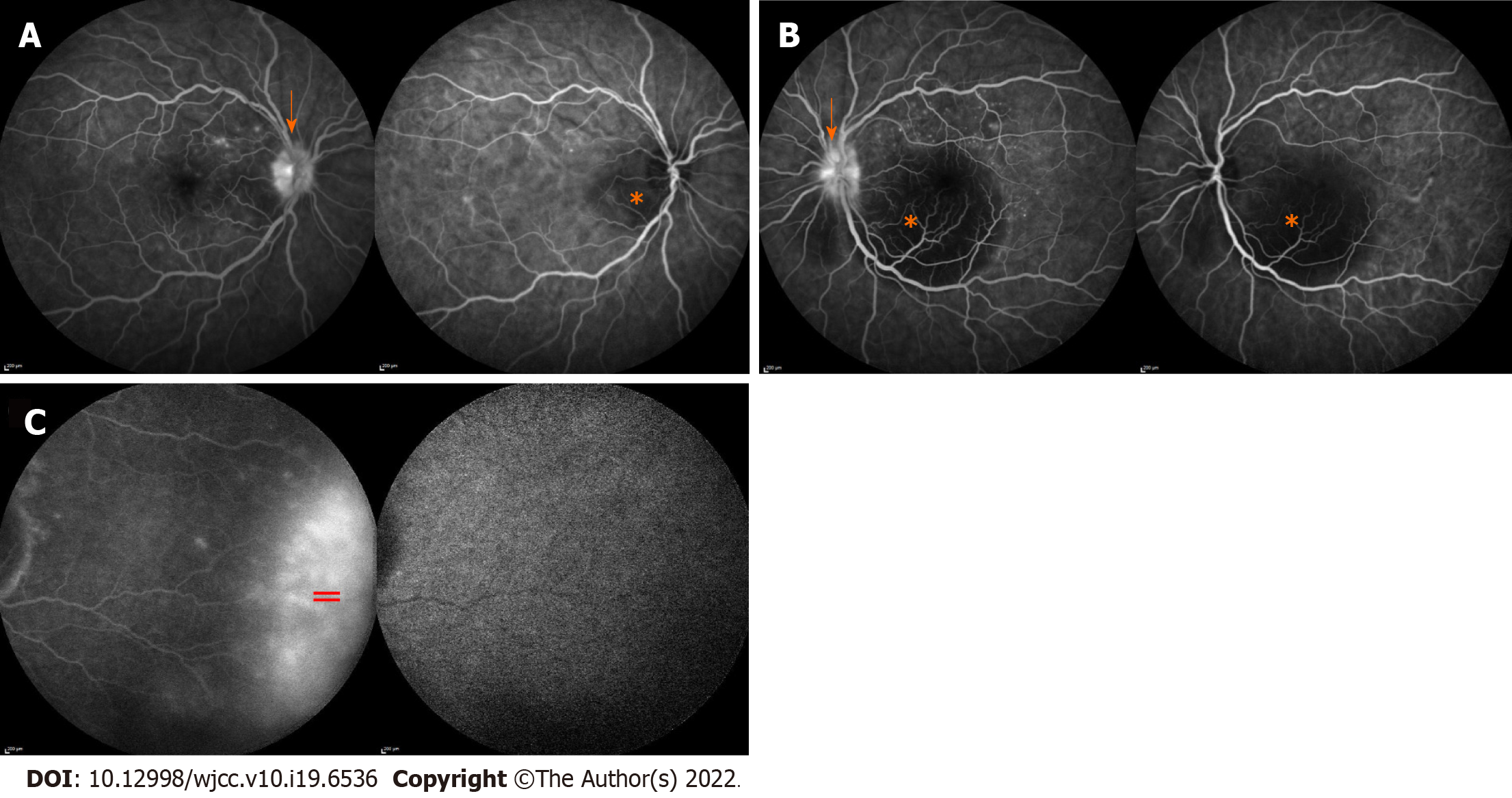
Optical coherence tomography (OCT) in the right eye revealed serous neurosensory retinal detachment temporal to the optic nerve disc, pachychoroid, and chorioretinal folds. OCT in the LE showed a subfoveal bullous serous exudative detachment with chorioretinal folds and pachychoroid (Figure 1). Fluorescein and indocyanine-green angiography revealed bilateral asymmetric (more in the LE) extensive leakage from the optic nerve discs and small hyperfluorescent spots at the upper temporal vascular arcade with leakage in the later phases. Moreover, in all four quadrants of the peripheral retina, extensive fluorescein leakage in later stages was observed (Figure 2) in both eyes. Echography revealed thickened choroid with lower internal reflectivity in both eyes, and serous retinal detachment in the LE. CT of the head and chest X-ray were unremarkable.
The final diagnosis of the presented case was bilateral immune checkpoint inhibitor-induced panuveitis.
Due to the history of recurrent herpetic keratouveitis, the patient was empirically treated with intravenous acyclovir, topical corticosteroids, and mydriatics at presentation. After a negative infectious uveitis work-up, the patient was treated with intravenous methylprednisolone (500 mg) for 3 d. Afterwards, steroid treatment was switched to oral methylprednisolone (64 mg) with a tapering schedule. Meanwhile, antiviral treatment with oral acyclovir (400 mg) twice daily was continued.
After the high dose systemic corticosteroid treatment with a tapering schedule, ocular morphological and functional outcomes improved. At the next follow-up, 1.5 mo after the ocular adverse effects onset, the patient’s best corrected visual acuity improved to 0.7 (RE) and 1.0 (LE). Inflammation in the anterior chamber and vitritis resolved. OCT revealed complete subretinal fluid reabsorption and chorioretinal fold resolution.
As the cause of the ocular changes was not clear, the MEK inhibitor was discontinued first; however, choroidal thickening and subretinal fluid reoccurred after the second cycle. Therefore, the BRAF inhibitor was discontinued and the MEK inhibitor was reintroduced, but the choroidal thickening and subretinal fluid persisted. Meanwhile, the patient was on low-dose peroral maintenance corticosteroid therapy. Because of the persistence of ocular adverse effects, BRAF/MEK inhibitor treatment was terminated, which resulted in the resolution of ocular adverse effects.
The patient continued with the regular follow-ups. After progression of the melanoma half a year later with the dissemination in the lungs, the BRAF/MEK inhibitors were reintroduced (vemurafenib and cobimetinib) along with peroral methylprednisolone (16 mg). At the time of writing this article, the patient’s ocular condition is unremarkable. She is on the second course of vemurafenib and cobimetinib treatment and her systemic condition is rapidly improving. The patient is not candidate for immunotherapy, as she developed grade 3 immune related pneumonitis.
Immunotherapy and targeted drugs therapy have recently become the new standard of care for patients with unresectable metastatic malignant melanoma, significantly improving the overall survival[3]. As with any new drug arrival on the market, the prevalence and severity of adverse effects are not well established. There are many published studies reporting ocular adverse effects associated with MEK or BRAF inhibitor monotherapy[5,6,11]; however, studies on combined therapy are lacking. To the best of our knowledge, there are only few published case reports reporting ocular adverse effects associated with dabrafenib and trametinib combined therapy[8-10]. Our case report closely resembles a case report published by Draganova et al[9], in which bilateral panuveitis, serous retinal detachment, chororetinal folds, and pachychoroid were described. In both case reports, dabrafenib and trametinib treatment was suspended, which resulted in significant functional and morphological improvement in 1-1.5 mo. After ruling out infectious causes of panuveitis, the patient was treated with intravenous corticosteroids (3 d) followed by oral corticosteroid treatment with a tapering schedule.
Because the patient had keratouveitis in the past and due to the immunosuppression with BRAF/MEK inhibitors, we started the treatment with oral acyclovir empirically at presentation. Although non-specific anterior uveitis and vitritis could be a sign of herpetic related panuveitis, multimodal imaging of the chorioretina excluded herpes simplex virus (HSV) involvement. In acute retinal necrosis (ARN) or progressive outer retinal necrosis (PORN), which are inflammatory conditions due to the varicella-zoster virus and HSV, necrotizing retinitis and occlusive arteritis are characteristic signs[12], which were not present in our patient. On the other hand, severe multiple subretinal serous retinal detachments and thick choroid, which were present in our patient, are not present in ARN and PORN. Serum testing for herpesvirus antibodies was not performed because it has been reported that it does not add any value in the diagnosis of ARN or PORN[12]. Of all blood tests, only Toxoplasma gondii IgG antibodies were positive. In adults, IgG antibodies against Toxoplasma gondii can be detected from 22.5% to more than 80% of people[13]. Due to the low diagnostic value of Toxoplasma gondii IgG antibodies and no other clinical signs suggesting ocular toxoplasmosis, this diagnosis was excluded.
The corticosteroid treatment paradigm for non-infectious uveitis is well established[14], which raises the question about corticosteroid treatment effectiveness in panuveitis induced by dabrafenib and trametinib. As described, after a consultation with our oncologist, we decided to discontinue the treatment with BRAF and MEK inhibitors, which resulted in the resolution of ocular adverse effects and metastatic melanoma dissemination. As seen in our case, stopping the BRAF and MEK inhibitor treatment can lead to melanoma dissemination, which can have an important impact on the survival. Since BRAF/MEK inhibitor treatment is the standard care for BRAF mutated melanoma patients, either in adjuvant or metastatic setting, we should be aware of possible ocular adverse effects including severe panuveitis. Moreover, due to the frequent ocular side effects of these medications, it might seem reasonable to schedule an ophthalmological examination for asymptomatic patients during the treatment. Along with the BRAF/MEK inhibitor treatment, patients could be treated with corticosteroids as well. The possibility of the adrenal gland suppression and other possible side effects of long-term corticosteroid treatment should be discussed with the patients.
The mechanism behind BRAF/MEK inhibitor induced panuveitis, which clinically closely resembles the Vogt-Koyanagi-Harada (VKH) disease[15], remains unclear. In the VKH disease pathogenesis, CD4+ and CD8+ cells (T cells) target melanocytic antigens in the choroid and RPE, which impair the outer blood retinal barrier[16]. BRAF/MEK inhibitors interfere with the MAPK pathway, which is involved in the T-cell receptor signaling pathway[17]. This interference could lead to similar changes in the choroid and RPE as observed in VKH disease[15].
In conclusion, MAPK pathway inhibition can very rarely lead to severe panuveitis in both eyes, which tends to resolve within months with treatment discontinuation and/or treatment with corticosteroids. Further studies are needed to determine optimal monitoring for these patients to exclude ocular adverse effects, and to establish treatment protocols if side effects occur.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Au SCL, China; Kapritsou M, Greece S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Grimaldi AM, Simeone E, Festino L, Vanella V, Strudel M, Ascierto PA. MEK Inhibitors in the Treatment of Metastatic Melanoma and Solid Tumors. Am J Clin Dermatol. 2017;18:745-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Simeone E, Grimaldi AM, Festino L, Vanella V, Palla M, Ascierto PA. Combination Treatment of Patients with BRAF-Mutant Melanoma: A New Standard of Care. BioDrugs. 2017;31:51-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1845] [Cited by in F6Publishing: 1839] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 4. | Heinzerling L, Eigentler TK, Fluck M, Hassel JC, Heller-Schenck D, Leipe J, Pauschinger M, Vogel A, Zimmer L, Gutzmer R. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open. 2019;4:e000491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Méndez-Martínez S, Calvo P, Ruiz-Moreno O, Pardiñas Barón N, Leciñena Bueno J, Gil Ruiz MDR, Pablo L. OCULAR ADVERSE EVENTS ASSOCIATED WITH MEK INHIBITORS. Retina. 2019;39:1435-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Choe CH, McArthur GA, Caro I, Kempen JH, Amaravadi RK. Ocular toxicity in BRAF mutant cutaneous melanoma patients treated with vemurafenib. Am J Ophthalmol. 2014;158:831-837.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Guedj M, Quéant A, Funck-Brentano E, Kramkimel N, Lellouch J, Monnet D, Longvert C, Gantzer A, Brézin AP. Uveitis in patients with late-stage cutaneous melanoma treated with vemurafenib. JAMA Ophthalmol. 2014;132:1421-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Albertini GC, Corbelli E, Battaglia Parodi M, Bandello F. Choroidal Neovascularization in Multifocal Choroiditis after Dabrafenib and Trametinib. Eur J Ophthalmol. 2017;27:e184-e186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Draganova D, Kerger J, Caspers L, Willermain F. Severe bilateral panuveitis during melanoma treatment by Dabrafenib and Trametinib. J Ophthalmic Inflamm Infect. 2015;5:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Joshi L, Karydis A, Gemenetzi M, Shao EH, Taylor SR. Uveitis as a Result of MAP Kinase Pathway Inhibition. Case Rep Ophthalmol. 2013;4:279-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Francis JH, Habib LA, Abramson DH, Yannuzzi LA, Heinemann M, Gounder MM, Grisham RN, Postow MA, Shoushtari AN, Chi P, Segal NH, Yaeger R, Ho AL, Chapman PB, Catalanotti F. Clinical and Morphologic Characteristics of MEK Inhibitor-Associated Retinopathy: Differences from Central Serous Chorioretinopathy. Ophthalmology. 2017;124:1788-1798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Powell B, Wang D, Llop S, Rosen RB. Management Strategies of Acute Retinal Necrosis: Current Perspectives. Clin Ophthalmol. 2020;14:1931-1943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Garweg JG, de Groot-Mijnes JD, Montoya JG. Diagnostic approach to ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19:255-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Kim JS, Knickelbein JE, Nussenblatt RB, Sen HN. Clinical trials in noninfectious uveitis. Int Ophthalmol Clin. 2015;55:79-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Joye A, Suhler E. Vogt-Koyanagi-Harada disease. Curr Opin Ophthalmol. 2021;32:574-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Crosson JN, Laird PW, Debiec M, Bergstrom CS, Lawson DH, Yeh S. Vogt-Koyanagi-Harada-like syndrome after CTLA-4 inhibition with ipilimumab for metastatic melanoma. J Immunother. 2015;38:80-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Proietti I, Skroza N, Michelini S, Mambrin A, Balduzzi V, Bernardini N, Marchesiello A, Tolino E, Volpe S, Maddalena P, Di Fraia M, Mangino G, Romeo G, Potenza C. BRAF Inhibitors: Molecular Targeting and Immunomodulatory Actions. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |