Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5646
Peer-review started: November 4, 2021
First decision: March 3, 2022
Revised: March 16, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Laparoscopic partial nephrectomy has been widely used in renal cell carcinoma treatment. The efficacy of GreenLight laser on Laparoscopic partial nephrectomy is still unknown.
To present the first series of laparoscopic partial nephrectomy (LPN) by GreenLight laser enucleation without renal artery clamping. Due to the excellent coagulation and hemostatic properties of the laser, laser-assisted LPN (LLPN) makes it possible to perform a “zero ischemia” resection.
Fifteen patients with T1a exogenous renal tumors who received high-power GreenLight laser non-ischemic LPN in our hospital were retrospectively analyzed. All clinical information, surgical and post-operative data, complications, pathological and functional outcomes were analyzed.
Surgery was successfully completed in all patients, and no open or radical nephrectomy was performed. The renal artery was not clamped, leading to no ischemic time. No blood transfusions were required, the average hemoglobin level ranged from 96.0 to 132.0 g/L and no postoperative complications occurred. The mean operation time was 104.3 ± 8.2 min. The postoperative removal of negative pressure drainage time ranged from 5.0 to 7.0 d, and the mean postoperative hospital stay was 6.5 ± 0.7 d. No serious complications occurred. Postoperative pathological results showed clear cell carcinoma in 12 patients, papillary renal cell carcinoma in 2 patients, and hamartoma in 1 patient. The mean creatinine level was 75.0 ± 0.8 μmol/L (range 61.0-90.4 μmol/L) at 1 mo after surgery, and there were no statistically significant differences compared with pre-operation (P > 0.05). The glomerular filtration rate ranged from 45.1 to 60.8 mL/min, with an average of 54.0 ± 5.0 mL/min, and these levels were not significantly different from those before surgery (P > 0.05).
GreenLight laser has extraordinary cutting and sealing advantages when used for small renal tumors (exogenous tumors of stage T1a) during LPN. However, use of this technique can lead to the generation of excessive smoke.
Core Tip: GreenLight laser has extraordinary cutting and sealing advantages when applied to exogenous T1a tumors during laparoscopic partial nephrectomy; GreenLight reduced the substantial sutures; GreenLight could lead to excessive smoke.
- Citation: Zhang XM, Xu JD, Lv JM, Pan XW, Cao JW, Chu J, Cui XG. “Zero ischemia” laparoscopic partial nephrectomy by high-power GreenLight laser enucleation for renal carcinoma: A single-center experience. World J Clin Cases 2022; 10(17): 5646-5654
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5646.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5646
Kidney cancer is a common tumor, accounting for 2%-3% of all carcinomas, and is one of the top 10 cancers worldwide[1]. Recent years have witnessed a consistent increase in the incidence rate in most countries[2]. To date, surgical therapy is still the primary treatment, especially in patients with a small renal mass (SRM), although surveillance is under study. Recent guidelines indicate that, as far as possible, all patients with tumors < 7 cm should receive nephron-sparing surgery (NSS). The disease-specific prognosis is similar between radical nephrectomy and partial nephrectomy (PN), with the benefit of better protection of kidney function in PN patients[3]. Thus, a critical target of NSS is to preserve the maximum amount of kidney parenchyma, with minimum warm ischemia time (WIT). Hilar clamping has been standard practice in previous decades to achieve the lowest blood loss. However, blockage of the renal blood supply results in WIT, and even renal function damage[4]. Bleeding is still the most frequent complication of NSS, with a risk of transfusion in up to 5% of patients[5]. Optimization of Renal cell carcinoma surgical treatment has received increased research interest. Over the years, progress has been made in reducing the risk of bleeding and the complications of WIT. From open to laparoscopic partial nephrectomy (LPN), therapy has recently changed to robot auxiliary partial nephrectomy[6]. Patients receiving laparoscopic surgery had lower intraoperative blood loss than those receiving the open surgery, and postoperative complications did not increase[7]. However, LPN prolonged WIT as the procedure is challenging even for experienced surgeons with critical time scales[8]. Thus, various techniques to reduce or eliminate WIT surgery have been used, including specific kidney artery block, targeted kidney blood flow or renal parenchyma clamping, laser-assisted minimal invasive partial nephrectomy (MIPN), MIPN auxiliary radio frequency, MIPN auxiliary water jet, and sequential preset kidney suture[9]. Although these techniques are not widely accepted, their applications are being increasingly investigated.
The initial GreenLight laser used potassium titanoxate phosphate, which produced a green visible light beam at 532 nm with a short penetration depth of 0.8 mm. GreenLight is selectively absorbed by hemoglobin rather than water within the tissue. The laser works by photoselective vaporization of tissues. This was followed by the development of a high-power 120W (GreenLight HPS) laser and, finally, the development of an 180W GreenLight accelerated performance system (XPS) laser with a Moxy fiber. The power of the laser and the laser beam area is increased by 50%, and the energy density at the laser point is similar, thus maintaining similar safety to the previous 120W system[10]. However, the GreenLight laser was widely used in transurethral resection of the prostate (TURP) for lower urinary tract symptoms associated with benign prostate enlargement, which led to the introduction of less invasive treatments. Although there have been animal experiments and pre-clinical investigations on GreenLight laser for NSS[11,12], the safety and feasibility of this technique in human NSS is unclear.
As different types of lasers have been tested, the purpose of this study was to demonstrate the feasibility of GreenLight (high-power 80-100W) laser-assisted LPN (LLPN). When oncology results and actual care standards match, tumor excision ability and the pathological report after laser excision are important.
From February 2021 to June 2021, 15 patients with localized exogenous kidney tumors were retrospectively analyzed. All patients received GreenLight laser surgery for NSS at the Gongli Hospital of Second Military Medical University. The surgical procedure and ethics were authorized by the Scientific Research Review Board of Gongli Hospital of Second Military Medical University. According to the results of imaging data, both the diagnosis of SRM and the surgical decision were made. To assess the complexity of the intervention, all patients were evaluated according to PADUA and R.E.N.A.L scores[6,13]. In the present study, only patients with a single lesion were included, with a maximum renal mass of 4 cm. Patients with centrally located tumors and with a single functional kidney were excluded. Ten males and 5 females, aged 47.0-74.0 years, with an average age of 58.6 ± 9.2 years were included. The tumor diameter ranged from 2.0 to 3.8 cm (3.0 ± 0.56 cm), 8 tumors on the left side and 7 on the right side, 14 on the dorsal side, and 1 on the ventral side. The preoperative glomerular filtration rate (GFR) on the diseased side was 44.6-67.3 mL/min (56.3 ± 6.8 mL/min). The preoperative hemoglobin level was 119.0-156.0 g/L, with an average value of 135.4 ± 10.8 g/L. All patients had exogenous renal space-occupying lesions on physical examination, and were diagnosed with renal carcinoma by renal artery enhanced CT examination before surgery. The R.E.N.A.L scores ranged from 4.0 to 6.0 (4.9 ± 0.8). One case was complicated with hypertension, one with coronary heart disease, and two with diabetes.
The targeted kidney SRMs were removed by zero-ischemic LLPN under the 180W XPS green laser system with a wavelength of 532 nm. The left foot set for the steam power of 80-100W, created a continuous launch mode. The hemostatic power of the right foot was 30-35W, and this was the simulated pulse emission mode. A Green laser fiber with active cooling cap technology was used. Retroperitoneal and transabdominal LPN was selected depending on the location and size of the patient's tumor. The patient was placed in the lateral decubitus position under general anesthesia, and the lumbar bridge was elevated.
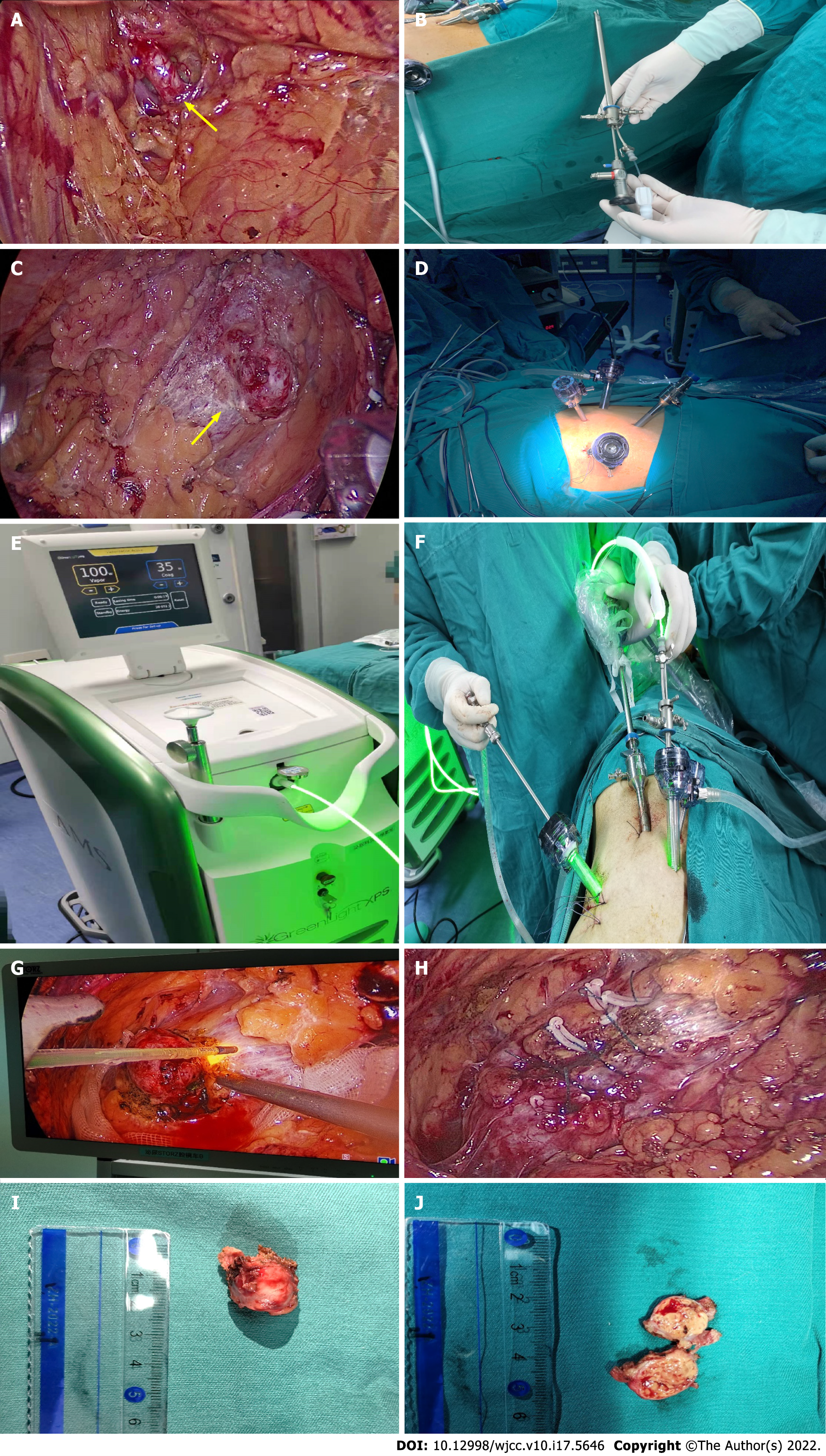
We applied continuous waves during the entire procedure. To perform laparoscopy, a flexible laser fiber with a beam of light was placed in the laparoscopic instrument. The procedure was conducted laparoscopically via retroperitoneal access. All procedures were performed by the same experienced surgeon. Small incisions were made in the 12th subcostal area of the posterior axillary line, the subcostal arch of the anterior axillary line, and 2 cm above the iliac crest of the mid-axillary line. The extra-peritoneal fat was removed, the lateral cone fascia was opened, and the renal artery was separated along the dorsal side of the kidney for reserve (Figure 1A). In the transabdominal approach, trocars were placed 3 cm above the umbilicus at the lateral margin of the rectus abdominis, 2 cm below the costal margin at the midline of the clavicle, and 3 cm above the internal anterior superior iliac spine. The lateral peritoneum at the para-colonic sulcus was opened to move the intestine downward. Dissociated along the pedicle direction, the renal vein was observed, separated and exposed, and the renal artery on the deep surface of the renal vein for reserve (no dissociation of the renal artery on the superficial surface of the tumor due to the small size of the tumor in 2 patients). Depending on the location of the tumor, the surrounding area was fully isolated and the renal tumor was completely exposed (Figure 1B). The laser fiber was inserted into the trocar through the green laser hand (Figure 1C and D), the fiber was connected to normal saline to wash the strips, the initial green laser steam power was set at 80W, and the hemostasis power was set at 35W (Figure 1E). The renal parenchyma was cut with 80W power (Figure 1F and G) at the height of one optical fiber head from the edge of the tumor approximately 3 mm from the renal parenchyma. When vaporizing, interference due to smoke was reduced by high pressure flushing of the optical fiber, and the tumor was pushed and stripped by the aspirator. In the case of intraoperative bleeding, the hemostatic power was used to seal the bleeding point (the power can be increased for large blood vessel bleeding), and the power was gradually increased to 80-100W according to the status of the evaporated kidney tissue. Progression was gradual until the tumor was completely removed. The wound surface of the inner medulla and outer cortex of the kidney were continuously sutured with 1-3 Layers of barb sutures (Figure 1H). The specimen bag was placed, the surgical area was flushed, the wound was checked for no active bleeding, a drainage tube was placed, the specimen was removed (Figure 1I and J) and the incision was sutured. All specimens were placed in formalin and histologically examined by pathologists. Drainage was inserted by default. Postoperative treatment was in accordance with our standard surgical procedures. The patients were followed up for 6 mo. The clinical manifestations and imaging findings were used to determine recurrence after surgery.
GraphPad Prism 7.00 was used for statistical analysis. The mean ± SD (numerical range) was used for statistical description of the data. The paired t-test was used for comparisons between preoperative and postoperative measurement data. The difference was considered statistically significant if the P value was less than 0.05.
Due to severe intraoperative bleeding, one patient underwent laparoscopic scissors rapid resection, and suturing to stop the bleeding. None of the patients were converted to open surgery or radical nephrectomy. The operative time ranged from 90.0 to 120.0 min, with an average time of 104.3 ± 8.2 min. The postoperative hemoglobin level was 96.0-132.0 g/L (114.9 ± 11.2 g/L), which was statistically significant compared with that before surgery (P < 0.05). The postoperative hemoglobin level decreased and ranged from 12.0 to 25.0 g/L. The average drainage time was 5.7 ± 0.7 d (5.0-7.0 d). The postoperative hospital stay ranged from 5.0 to 8.0 d (6.7 ± 0.7 d). No serious complications occurred in these patients. One patient had hypertension, one patient had coronary heart disease, and two patients had diabetes. One month after the operation, the creatinine was 61.0-90.4 μmol/L (75.0 ± 8.5 μmol/L), which was not significant compared with that before surgery (P > 0.05). The GFR on the affected side was evaluated one month after surgery, and the average value was 54.0 ± 5.0 mL/min. There was no significant difference between preoperative and preoperative levels (P > 0.05). No tumor recurrence or metastasis was observed during the short-term follow-up period.
It is standard procedure to perform laparoscopic surgery for the removal of renal tumors and SRMs, with renal artery clamping and WIT. However, the difficulty and requirements of LPN lead to longer WIT, compared with the open method. Moreover, WIT deserves more attention in the case of pre-damaged organs or a single kidney[8]. More recently, robot-assisted LPN has emerged as an alternative to LPN. Compared with laparoscopic surgery, the ischemia time during robotic surgery is significantly shortened, thereby reducing the risk of renal dysfunction[14]. Current studies have shown that renal artery clamping for more than 30 min can cause irreversible renal function damage[15]. Recently, Thompson et al[16] proposed that as long as the blood supply to the kidney is blocked, kidney damage will gradually increase every minute. These findings present a significant challenge to urologists in how best to preserve renal function in patients with early renal tumors. Therefore, a complete unblocked nephrectomy of the renal artery is necessary. This technique was first reported by Marshall et al[17] and Abaza et al[18] in 2000. Surgeons performed PN without blood vessel clamping in patients with SRMs using hemostasis devices such as double-click electro-coagulation[18]. It seems that decreasing WIT could be a favorable modifiable risk factor to avoid postoperative kidney dysfunction. Thus, we attempted to investigate the safety and feasibility of the GreenLight laser, and to reduce or prevent WIT in renal laparoscopic surgery.
The application of a laser during kidney surgery remains uncertain in the experimental or preclinical stage, although it has been widely used and extensively investigated in other medical areas. As the laser's efficacy depends on the wavelength and the proportion of water in the tissue, its efficacy must be determined. The feasibility of using lasers in kidney procedures has been shown previously[19-21]. Kyriazis et al[19] first reported 2 cases of unblocked thulium laser-assisted robotic PN with no obvious intraoperative or postoperative complications, and the pathological results showed a negative surgical margin. Boris et al[22] successfully carried out green laser partial zero ischemia nephrectomy in pigs, and the results showed that the green laser effectively stop bleeding within a very short time, with a tissue penetration rate of only 0.8 mm. The GreenLight laser applied in the present study at 532 nm, was preferentially absorbed by oxyhemoglobin (absorption coefficient 102/cm), but not by rinsing (absorption coefficient 104/cm). The increased energy absorbed from hemoglobin caused the tissue to vaporize, leading to physical separation of the tissue. In addition, it resulted in thermally-induced coagulation of superficial blood vessels; thus, an almost blood-free area was produced for surgery. The 532 nm wavelength has a small penetration depth (1-2 mm) leading to less charring[15,23,24]. Thus, GreenLight laser vaporization is considered an effective alternative for TURP. Based on this, we attempted to demonstrate the balance between laser energy and NSS.
In this study involving 15 patients, no obvious complications such as urine leakage and bleeding occurred during the perioperative period. The postoperative follow-up examination indicated that no patient had positive surgical margins or postoperative local recurrence, and there were no significant statistical differences between preoperative and postoperative serum creatinine levels. LPN is safe, feasible, and beneficial for maximum preservation of renal function in patients. To our knowledge, this is the first report on GreenLight LLPN to date. Moreover, all the tumors were removed without WIT, which protected kidney function. However, one of the major limitations of surgery was the excessive accumulation of smoke during vaporization of tissue. We attempted to reduce this by rinsing, but visibility was not improved. Favorable visibility could be obtained by using one of the trocars as a fume hood. However, this was not the best option as better visibility could not be acquired from suction alone. In addition, it appears that undefined resection margins and positive resection margins are an issue on histopathological examination. Nevertheless, this should be validated in further large-cohort studies. All patients with unclear or positive surgical margins were followed up and no tumor recurrence has been observed.
The potential efficacy of laser-assisted LPN without WIT has been witnessed for over a decade[25], but until now this technical option has been considered experimental. In this retrospective study, which consisted of the first series of patients to date, we showed the strengths and main problems of GreenLight (80-100W) LLPN. However, this technique is mainly limited to single cases with a small tumor volume and superficial locations. The number of cases in the present study was small; thus, further clinical trials are required to determine whether this technique should be promoted.
Laparoscopic partial nephrectomy has been widely used in renal cell carcinoma treatment. The efficacy of GreenLight laser on Laparoscopic partial nephrectomy is still unknown. To present the first series of laparoscopic partial nephrectomy (LPN) by GreenLight laser (KTP) enucleation without renal artery clamping. Due to the excellent coagulation and hemostatic properties of the laser, laser-assisted LPN (LLPN) makes it possible to perform a “zero ischemia” resection.
To date, surgical therapy is still the primary treatment, especially in patients with a small renal mass, although surveillance is under study. Recent guidelines indicate that, as far as possible, all patients with tumors < 7 cm should receive nephron-sparing surgery (NSS). The disease-specific prognosis is similar between radical nephrectomy and partial nephrectomy (PN), with the benefit of better protection of kidney function in PN patients. Thus, a critical target of NSS is to preserve the maximum amount of kidney parenchyma, with minimum warm ischemia time (WIT). Hilar clamping has been standard practice in previous decades to achieve the lowest blood loss. However, blockage of the renal blood supply results in WIT, and even renal function damage. Bleeding is still the most frequent complication of NSS, with a risk of transfusion in up to 5% of patients.
To present the first series of LPN by GreenLight laser enucleation without renal artery clamping. Due to the excellent coagulation and hemostatic properties of the laser, LLPN makes it possible to perform a “zero ischemia” resection.
Fifteen patients with T1a exogenous renal tumors who received high-power GreenLight laser non-ischemic LPN in our hospital were retrospectively analyzed. All clinical information, surgical and post-operative data, complications, pathological and functional outcomes were analyzed.
Surgery was successfully completed in all patients, and no open or radical nephrectomy was performed. The renal artery was not clamped, leading to no ischemic time. No blood transfusions were required, the average hemoglobin level ranged from 96.0 to 132.0 g/L and no postoperative complications occurred. The mean operation time was 104.3 ± 8.2 min. The postoperative removal of negative pressure drainage time ranged from 5.0 to 7.0 d, and the mean postoperative hospital stay was 6.5 ± 0.7 d. No serious complications occurred. Postoperative pathological results showed clear cell carcinoma in 12 patients, papillary renal cell carcinoma in 2 patients, and hamartoma in 1 patient. The mean creatinine level was 75.0 ± 0.8 μmol/L (range 61.0-90.4 μmol/L) at 1 mo after surgery, and there were no statistically significant differences compared with pre-operation (P > 0.05). The glomerular filtration rate ranged from 45.1 to 60.8 mL/min, with an average of 54.0 ± 5.0 mL/min, and these levels were not significantly different from those before surgery (P > 0.05).
GreenLight laser has extraordinary cutting and sealing advantages when used for small renal tumors (exogenous tumors of stage T1a) during LPN. However, use of this technique can lead to the generation of excessive smoke.
GreenLight laser has extraordinary cutting and sealing advantages when applied to exogenous T1a tumors during LPN; GreenLight reduced the substantial sutures; GreenLight could lead to excessive smoke.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salimi M, Iran S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 51049] [Article Influence: 8508.2] [Reference Citation Analysis (122)] |
| 2. | Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 434] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 3. | MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, Royle P, Stewart F, MacLennan G, MacLennan SJ, Canfield SE, McClinton S, Griffiths TR, Ljungberg B, N'Dow J; UCAN Systematic Review Reference Group; EAU Renal Cancer Guideline Panel. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61:972-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | Volpe A, Blute ML, Ficarra V, Gill IS, Kutikov A, Porpiglia F, Rogers C, Touijer KA, Van Poppel H, Thompson RH. Renal Ischemia and Function After Partial Nephrectomy: A Collaborative Review of the Literature. Eur Urol. 2015;68:61-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 5. | Gratzke C, Seitz M, Bayrle F, Schlenker B, Bastian PJ, Haseke N, Bader M, Tilki D, Roosen A, Karl A, Reich O, Khoder WY, Wyler S, Stief CG, Staehler M, Bachmann A. Quality of life and perioperative outcomes after retroperitoneoscopic radical nephrectomy (RN), open RN and nephron-sparing surgery in patients with renal cell carcinoma. BJU Int. 2009;104:470-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Laird A, Choy KC, Delaney H, Cutress ML, O'Connor KM, Tolley DA, McNeill SA, Stewart GD, Riddick AC. Matched pair analysis of laparoscopic versus open radical nephrectomy for the treatment of T3 renal cell carcinoma. World J Urol. 2015;33:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Gong EM, Orvieto MA, Zorn KC, Lucioni A, Steinberg GD, Shalhav AL. Comparison of laparoscopic and open partial nephrectomy in clinical T1a renal tumors. J Endourol. 2008;22:953-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Muramaki M, Miyake H, Sakai I, Fujisawa M. Prognostic Factors Influencing Postoperative Development of Chronic Kidney Disease in Patients with Small Renal Tumors who Underwent Partial Nephrectomy. Curr Urol. 2013;6:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Hou W, Ji Z. Achieving zero ischemia in minimally invasive partial nephrectomy surgery. Int J Surg. 2015;18:48-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Gomez-Sancha F. GreenLight laser vaporization of the prostate: has it come of age? Curr Opin Urol. 2015;25:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Hindley RG, Barber NJ, Walsh K, Petersen A, Poulsen J, Muir GH. Laparoscopic partial nephrectomy using the potassium titanyl phosphate laser in a porcine model. Urology. 2006;67:1079-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Eret V, Hora M, Sykora R, Hes O, Urge T, Klecka J, Matejovic M. GreenLight (532 nm) laser partial nephrectomy followed by suturing of collecting system without renal hilar clamping in porcine model. Urology. 2009;73:1115-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Drerup M, Magdy A, Hager M, Colleselli D, Kunit T, Lusuardi L, Janetschek G, Mitterberger M. Non-ischemic laparoscopic partial nephrectomy using 1318-nm diode laser for small exophytic renal tumors. BMC Urol. 2018;18:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, Wang AJ, Stifelman MD. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 15. | Barber NJ, Muir GH. High-power KTP laser prostatectomy: the new challenge to transurethral resection of the prostate. Curr Opin Urol. 2004;14:21-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, Gill IS, Blute ML, Campbell SC. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58:340-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 510] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 17. | Marshall FF. Laparoscopic nephron-sparing surgery for renal tumors. J Urol. 2002;168:876. [PubMed] [Cited in This Article: ] |
| 18. | Abaza R, Picard J. A novel technique for laparoscopic or robotic partial nephrectomy: feasibility study. J Endourol. 2008;22:1715-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Kyriazis I, Ozsoy M, Kallidonis P, Panagopoulos V, Vasilas M, Liatsikos E. Current evidence on lasers in laparoscopy: partial nephrectomy. World J Urol. 2015;33:589-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Korhonen AK, Talja M, Karlsson H, Tuhkanen K. Contact Nd:YAG laser and regional renal hypothermia in partial nephrectomy. Ann Chir Gynaecol Suppl. 1993;206:59-62. [PubMed] [Cited in This Article: ] |
| 21. | Zhou XF, Ding ZS, Wang JF, Chen X, Fang ZL, Liu NB, Zhang G, Zhao PY. Laparoscopic Partial Nephrectomy by Diode Laser with Highly Selective Clamping of Segmental Renal Arterial. Chin Med J (Engl). 2015;128:2262-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Boris RS, Eun D, Bhandari A, Lyall K, Bhandari M, Rogers C, Alassi O, Menon M. Potassium-titanyl-phosphate laser assisted robotic partial nephrectomy in a porcine model: can robotic assistance optimize the power needed for effective cutting and hemostasis? J Robot Surg. 2007;1:185-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Van Cleynenbreugel B, Srirangam SJ, Van Poppel H. High-performance system GreenLight laser: indications and outcomes. Curr Opin Urol. 2009;19:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Benejam Gual J, Servera Ruiz de Velasco A, Hernández Martínez Y, García-Miralles Grávalos R. [Greenlight laser vaporization: Past, present and future? Arch Esp Urol. 2020;73:675-681. [PubMed] [Cited in This Article: ] |
| 25. | Malloy TR, Schultz RE, Wein AJ, Carpiniello VL. Renal preservation utilizing neodymium:YAG laser. Urology. 1986;27:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |