Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5634
Peer-review started: December 27, 2021
First decision: January 25, 2022
Revised: February 11, 2022
Accepted: April 9, 2022
Article in press: April 29, 2022
Published online: June 16, 2022
Colon and rectal cancers are among the top five cancers worldwide in terms of their incidence and mortality rates. As the treatment options for cure include surgery even in specific advanced-stage cases, the early detection of lesions is important for applying active treatment methods. Fluorine-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography/computed tomography (PET/CT) is an established imaging study for many types of cancers; however, physiologic uptake in the gastrointestinal tract is a frequent finding and may interfere with lesion identification. Nevertheless, as unexpectedly observed focal colorectal F-18 FDG uptake may harbor malignant lesions, further examination must not be avoided.
To assess the clinical implications of unexpected focal colorectal F-18 FDG uptake by analyzing FDG PET parameters.
A total of 15143 F-18 FDG PET/CT scans performed at our hospital between January 2016 and September 2021 were retrospectively reviewed to identify incidentally observed focal colorectal FDG uptake. Finally, 83 regions showing focal colorectal FDG uptake with final histopathological reports from 80 patients (45 men and 35 women with mean ages of 66.9 ± 10.7 years and 63.7 ± 15.3 years, respectively) were eligible for inclusion in the present study. Each focal hypermetabolic colorectal region was classified as malignant, premalignant, or benign according to the histopathological report. PET parameters such as maximum and peak standardized uptake value (SUVmax and SUVpeak), metabolic tumor volume (MTV), mean SUV of the metabolic tumor volume (mSUVmtv), and total lesion glycolysis (TLG) were measured or calculated for the corresponding hypermetabolic regions. Parametric and non-parametric statistical comparisons of these parameters were performed among the three groups. Receiver operating characteristic curves were plotted to identify cut-off values.
The detection rate of incidental focal colorectal uptake was 0.53% (80/15,143). Of the 83 regions with unexpected focal colorectal hypermetabolism, 28.9% (24/83) were malignant, 32.5% (27/83) were premalignant, and 38.6% (32/83) were benign. Overall, 61.4% of the regions had malignant or premalignant lesions. SUVmax, SUVpeak, and mSUVmtv differentiated malignant and/or premalignant lesions from benign lesions with statistical significance (P < 0.05). mSUVmtv3.5 differentiated malignant from benign lesions, with the largest area under the curve (AUC) of 0.792 and a cut-off of 4.9. SUVmax showed the largest AUC of 0.758 with a cut-off value of 7.5 for distinguishing between premalignant and benign lesions. Overall, SUVmax with a cut-off value of 7.6 (AUC: 0.770, 95% confidence interval (CI): 0.668-0.872; sensitivity, 0.686; specificity, 0.688) was a superior parameter for distinguishing between malignant/premalignant and benign lesions or physiologic uptake. No parameters differentiated malignant from premalignant lesions. Moderate or weak positive correlations were observed between the long diameter of the malignant lesions and PET parameters such as SUVpeak and some mSUVmtv.
Approximately two-thirds (61.4%) of incidental focal hypermetabolic colorectal regions were malignant/premalignant lesions, for which SUVmax was an independent diagnostic parameter. Unexpected suspicious focal colorectal FDG uptake should not be avoided and consideration for further evaluation is strongly recommended not to miss the two-thirds.
Core Tip: Although intestinal fluorine-18 fluorodeoxyglucose (F-18 FDG) uptake is not a rare finding on F-18 FDG positron emission tomography/computed tomography, focal colorectal uptake may harbor malignant lesions. Therefore, it is important to differentiate between malignant/premalignant and benign lesions. The present study compared PET parameters such as standardized uptake value (SUV), metabolic tumor volume, mean SUV of metabolic tumor volume, and total lesion glycolysis among the malignant, premalignant, and benign incidental focal hypermetabolism to evaluate the implications of unexpectedly observed focal colorectal FDG uptake.
- Citation: Lee H, Hwang KH, Kwon KA. Assessment of incidental focal colorectal uptake by analysis of fluorine-18 fluorodeoxyglucose positron emission tomography parameters. World J Clin Cases 2022; 10(17): 5634-5645
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5634.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5634
According to the Global Cancer Observatory, the worldwide estimated age-standardized incidence and mortality rates of colorectal cancer for both sexes and all ages in 2020 were 19.5 (4th) and 9.0 (3rd), respectively[1,2], placing the disease among the top five leading cancers.
Like many other cancers, the treatment options for colorectal cancer include local or systemic treatments; however, surgery may be useful for cure in selected colorectal cancer patients with a limited number of small metastatic lesions (stage IV). Even in cases with large or many metastases, surgery may still be considered if the lesions shrink after neoadjuvant chemotherapy. In this way, more active treatment method could be a choice for colorectal cancer than for other cancers, and an improvement in overall survival may be expected through the early detection of lesions.
Fluorine-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography/computed tomography (PET/CT) is an established imaging modality used for the diagnosis, treatment response, and follow-up of many types of cancers. Physiologic gastrointestinal FDG uptake is well known, particularly in the colon and rectum, and diffusely or segmentally increased intestinal F-18 FDG uptake (hypermetabolism) is often observed as normal physiologic uptake[3-7]. This may obscure and interfere with the detection of true lesions. Despite this pitfall, FDG PET/CT may help detect lesions that are malignant or harbor a risk of malignancy, which appear as incidentally visualized focal FDG uptake in the intestines[8-10]. This retrospective study aimed to identify the implications of unexpectedly observed focal colorectal hypermetabolism on F-18 FDG PET/CT performed for purposes other than colorectal concerns by comparing PET parameters among histopathologically confirmed malignant, premalignant, and benign focal hypermetabolism.
To identify incidental focal colorectal hypermetabolic lesions, we retrospectively reviewed 15,143 F-18 FDG PET/CT scans performed at our hospital between January 2016 and September 2021. After excluding the scans of patients with current or prior colorectal malignancies or without histopathological reports (gold standard) of the corresponding hypermetabolic regions, 80 patients (45 men and 35 women with mean age 66.9 ± 10.7 years and 63.7 ± 15.3 years, respectively) with 83 regions of focal colorectal FDG uptake and their final histopathological reports were eligible for this study.
To acquire images of F-18 FDG PET/CT with optimal image quality, all patients fasted for 4-6 h and their blood glucose levels were checked. The examination was rescheduled in cases with blood glucose levels ≥ 11 mmol/L (200 mg/dL). Scanning was performed 60 min after the intravenous injection of 185 MBq F-18 FDG. Images from the skull base to the upper thigh were acquired using a dedicated PET/CT scanner (Biograph mCT 128, Siemens Healthcare GmbH, Erlangen, Germany). Emission scans were acquired using the step-and-shoot method for 3 min per bed. CT scans were performed using the continuous spiral mode with CareDose4D and CARE kV activated to reduce patient radiation exposure and acquire individually optimized images. No contrast material was used for the CT scans. Both PET and CT images were reconstructed using the iterative reconstruction method and the final fused PET/CT images were generated on a dedicated image acquisition workstation provided with the PET/CT device.
Two nuclear medicine physicians, one with over 20 years of experience, reviewed the PET/CT images. When a region of focal abnormal FDG uptake by the colon and/or rectum was identified, the patient’s medical records were reviewed to obtain a histopathological report of the corresponding location, if available. The hypermetabolic regions revealed by the final histopathological reports, as well as on PET/CT, were categorized as malignant, premalignant, or benign. For these, semi-quantitative standardized uptake value (SUV) was measured as maximum (SUVmax) and peak (SUVpeak). In addition, the metabolic tumor volume (MTV) was measured, which provided information on both the volume and the mean SUV of the volume. When measuring the MTV, different volumes of interest can be applied using different settings of the SUV threshold. This study used several SUV thresholds, ranging from 2 to 5 in increments of 0.5, to obtain multiple MTVs and the mean SUV of each MTV with specific SUV threshold # (MTV# and mSUVmtv#, respectively). Finally, the total lesion glycolysis (TLG) # was calculated by multiplying the volume from the MTV# by the mSUVmtv#. All imaging analyses were performed using a dedicated PET/CT workstation equipped with SyngoMMWP (Siemens Healthcare GmbH, Erlangen, Germany). The measured and calculated PET parameters were compared among the malignant, premalignant, malignant/premalignant, and benign lesions, and receiver operating characteristic (ROC) curve analysis was performed to identify the cut-off values. Additionally, the correlations between PET parameters and tumor size (long diameter) were evaluated.
Both parametric (Student's t-test) and non-parametric (such as Mann–Whitney U test) methods were used to compare SUVmax, SUVpeak, MTV#, mSUVmtv#, and TLG# among the categorized lesions, and to correlate the parameters and size of malignant tumors. ROC curves were plotted and the areas under the curves (AUCs) were calculated to determine the optimal cut-off values to distinguish malignant and/or premalignant from benign lesions. Statistical analysis was performed using SPSS for Windows, version 16.0 (SPSS, Inc., Chicago, IL, United States). Statistical significance was set at P < 0.05.
This retrospective study was approved by the Institutional Review Board of our hospital (IRB no. GAIRB2020-297), and the requirement for informed consent was waived. The study was conducted in accordance with the 1964 Declaration of Helsinki and later amendments.
The demographic and clinical characteristics of the 80 patients classified by histopathological reports are shown in Table 1. The detection rate of incidental focal colorectal uptake was 0.53% (80/15,143). Among the 83 eligible regions of focal colorectal hypermetabolism, 24 were diagnosed as malignant lesions, 27 were premalignant, and the remaining 32 were benign. In terms of malignant lesions, they were 28.9% (24/83) of the focal hypermetabolic regions, consisting of 23 cases of adenocarcinoma and one case of neuroendocrine tumor. Premalignant lesions included tubular (77.8%, 21/27), villous (7.4%, 2/27), and tubulovillous (14.8%, 4/27) adenomas. The benign group comprised patients with inflammation or physiologic uptake with no remarkable mucosal abnormalities on colonoscopy. Overall, 61.4% (51/83) of the regions had malignant or premalignant lesions.
| Nature of incidental focal hypermetabolism | Characteristics | Men | Women | Total, % |
| Malignant | Subjects (n) | 16 | 8 | 24 |
| (lesions, n = 24) | Age (yr, mean ± SD) | 70.1 ± 11.5 | 72.5 ± 14.1 | 71 ± 12.1 |
| Primary malignancy (n) | ||||
| Lung | 5 | 0 | 5 (20.8) | |
| Stomach | 5 | 0 | 5 (20.8) | |
| Breast | 0 | 3 | 3 (12.5) | |
| Prostate | 1 | 0 | 1 (4.2) | |
| Lymphoma | 0 | 1 | 1 (4.2) | |
| Hepatobiliary | 2 | 1 | 3 (12.5) | |
| Other | 3 | 3 | 6 (25.0) | |
| Premalignant | Subjects (n) | 20 | 6 | 26 |
| (lesions, n = 27) | Age (yr, mean ± SD) | 67.9 ± 6.4 | 68.8 ± 18.7 | 68.1 ± 10.1 |
| Primary malignancy (n) | ||||
| Lung | 10 | 1 | 11 (42.3) | |
| Stomach | 4 | 1 | 5 (19.2) | |
| Breast | 0 | 0 | 0 (0.0) | |
| Prostate | 2 | 0 | 2 (7.7) | |
| Lymphoma | 1 | 1 | 2 (7.7) | |
| Hepatobiliary | 2 | 2 | 4 (15.4) | |
| Other | 1 | 1 | 2 (7.7) | |
| Malignant/ | Subjects (n) | 36 | 14 | 50 |
| Premalignant | Age (yr, mean ± SD) | 68.9 ± 8.94 | 70.9 ± 15.7 | 69.4 ± 11.1 |
| (lesions, n = 51) | Primary malignancy (n) | |||
| Lung | 15 | 1 | 16 (32.0) | |
| Stomach | 9 | 1 | 10 (20.0) | |
| Breast | 0 | 3 | 3 (6.0) | |
| Prostate | 3 | 0 | 3 (6.0) | |
| Lymphoma | 1 | 2 | 3 (6.0) | |
| Hepatobiliary | 4 | 3 | 7 (14.0) | |
| Other | 4 | 4 | 8 (16.0) | |
| Benign | Subjects (n) | 9 | 21 | 30 |
| (lesions, n = 32) | Age (yr, mean ± SD) | 58.9 ± 13.9 | 58.9 ± 13.3 | 58.9 ± 13.3 |
| Primary malignancy (n) | ||||
| Lung | 3 | 2 | 5 (16.7) | |
| Stomach | 3 | 5 | 8 (26.7) | |
| Breast | 0 | 4 | 4 (13.3) | |
| Prostate | 1 | 0 | 1 (3.3) | |
| Lymphoma | 0 | 1 | 1 (3.3) | |
| Hepatobiliary | 2 | 2 | 4 (13.3) | |
| Other | 0 | 7 | 7 (23.3) |
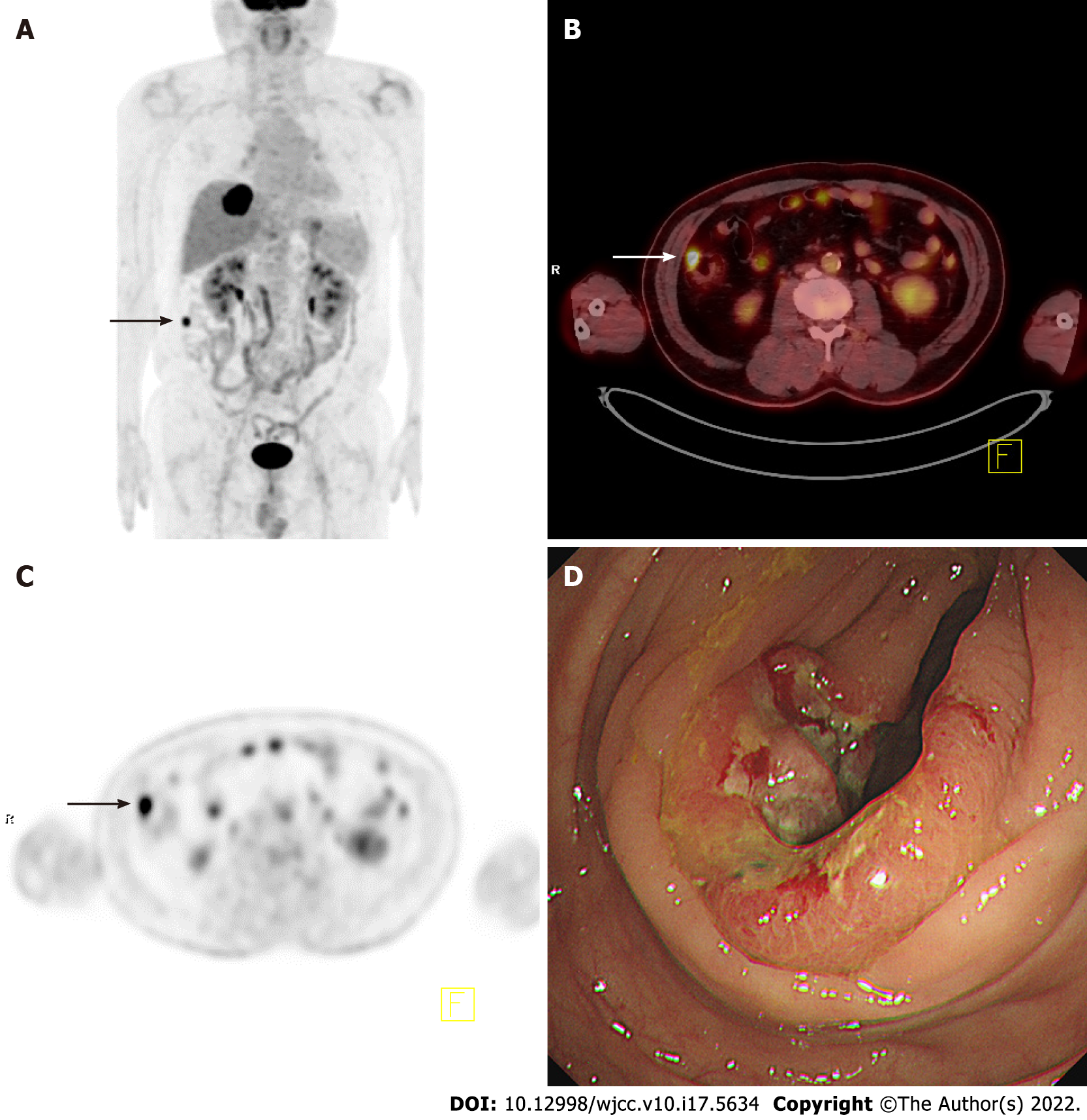
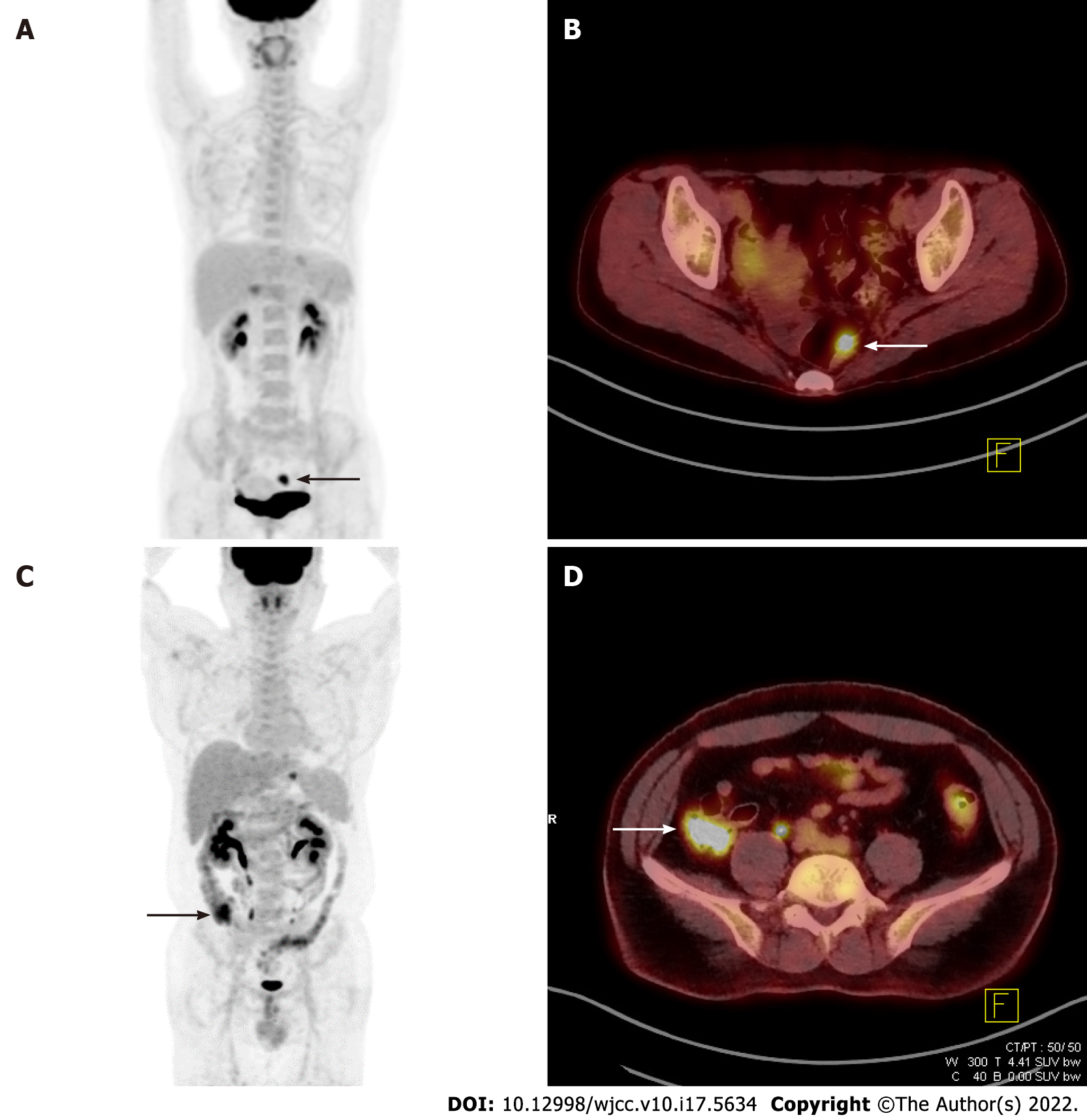
The five PET parameters considered in this study (SUVmax, SUVpeak, MTV#, mSUVmtv#, and TLG#) were compared among malignant, premalignant, malignant/premalignant, and benign lesions. Table 2 shows representative examples of these comparisons. SUVmax, SUVpeak, and all mSUVmtv# differed significantly between malignant and benign, premalignant and benign, and malignant/premalignant and benign lesions, while no parameters showed significant differences between malignant and premalignant lesions. Figure 1 shows an example of incidental focal ascending colon uptake, which was diagnosed as adenocarcinoma in a patient with a known intrahepatic cholangiocarcinoma. Figure 2 shows a patient with incidental focal rectal uptake (A and B) diagnosed as villous adenoma and a case of proximal ascending colon uptake (C and D) with no remarkable mucosal lesion revealed on colonoscopy.
| Malignant (n = 24) | Premalignant (n = 27) | P value | |
| mean SUVmax ± SD | 12.8 ± 7.6 | 10.5 ± 4.7 | > 0.05 |
| mean SUVpeak ± SD | 9.7 ± 6.1 | 7.9 ± 4.0 | > 0.05 |
| Malignant (n=24) | Benign (n=32) | P value | |
| mean SUVmax ± SD | 12.8 ± 7.6 | 7.2 ± 3.4 | < 0.05 |
| mean SUVpeak ± SD | 9.7 ± 6.1 | 5.6 ± 2.7 | < 0.05 |
| mean mSUVmtv3.5 ± SD | 6.1 ± 1.8 | 4.7 ± 0.8 | < 0.05 |
| Premalignant (n=27) | Benign (n=32) | P value | |
| mean SUVmax ± SD | 10.5 ± 4.7 | 7.2 ± 3.4 | < 0.05 |
| mean SUVpeak ± SD | 7.9 ± 4.0 | 5.6 ± 2.7 | < 0.05 |
| mean mSUVmtv4.5 ± SD | 6.5 ± 1.5 | 5.5 ± 0.9 | < 0.05 |
| Malignant/premalignant (n = 51) | Benign (n = 32) | P value | |
| mean SUVmax ± SD | 11.6 ± 6.3 | 7.2 ± 3.4 | < 0.05 |
| mean SUVpeak ± SD | 8.8 ± 5.1 | 5.6 ± 2.7 | < 0.05 |
| mean mSUVmtv3.5 ± SD | 5.9 ± 1.6 | 4.7 ± 0.8 | < 0.05 |
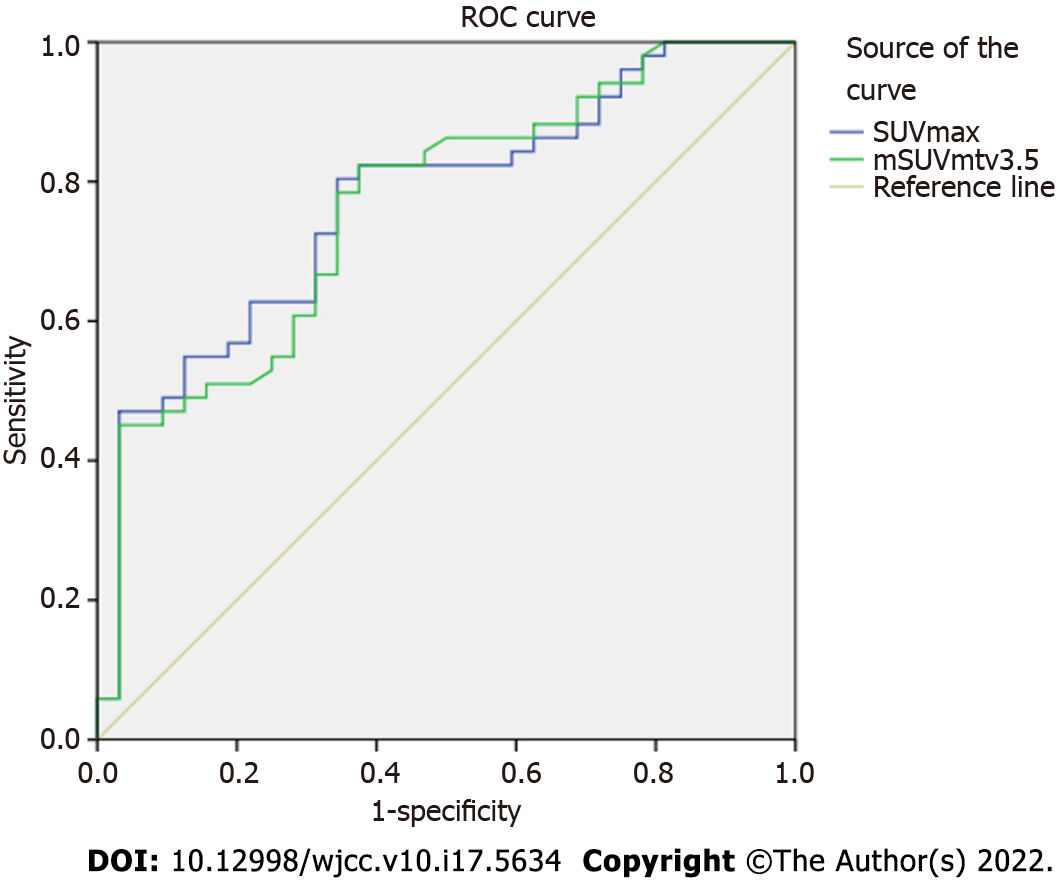
ROC curves were plotted, and cut-offs were determined for malignant, premalignant, and malignant/premalignant lesions. The AUC, cut-off, 95% confidence interval (CI), sensitivity, and specificity of each parameter are shown in Table 3. An AUC of 0.792 was calculated for mSUVmtv3.5 and a cut-off of 4.9 (CI, 0.671-0.914; sensitivity, 0.667; specificity, 0.656) differentiated malignant from benign lesions. An AUC of 0.758 was calculated for SUVmax, with a cut-off of 7.5 (CI, 0.634-0.882; sensitivity, 0.704; specificity, 0.688) distinguishing between premalignant and benign lesions. Likewise, an AUC 0.770 for SUVmax and a cut-off of 7.6 (CI, 0.668-0.872; sensitivity, 0.686; specificity, 0.688) differentiated malignant/premalignant from benign lesions. Figure 3 shows the ROC curves for SUVmax and mSUVmtv3.5 for malignant/premalignant lesions.
| Parameter | AUC | Cut-off | Confidence interval | Sensitivity | Specificity | |
| Malignant | SUVmax | 0.784 | 7.6 | 0.659 - 0.909 | 0.708 | 0.688 |
| SUVpeak | 0.767 | 5.9 | 0.640 - 0.894 | 0.708 | 0.656 | |
| mSUVmtv5 | 0.773 | 6.0 | 0.632 - 0.914 | 0.696 | 0.680 | |
| mSUVmtv4.5 | 0.778 | 5.6 | 0.647 - 0.909 | 0.667 | 0.667 | |
| mSUVmtv4 | 0.784 | 5.3 | 0.657 - 0.911 | 0.667 | 0.677 | |
| mSUVmtv3.5 | 0.792 | 4.9 | 0.671 - 0.914 | 0.667 | 0.656 | |
| mSUVmtv3 | 0.786 | 4.5 | 0.664 - 0.909 | 0.667 | 0.656 | |
| mSUVmtv2.5 | 0.775 | 4.1 | 0.649 - 0.902 | 0.625 | 0.656 | |
| mSUVmtv2 | 0.722 | 3.8 | 0.588 - 0.856 | 0.625 | 0.625 | |
| Premalignant | SUVmax | 0.758 | 7.5 | 0.634 - 0.882 | 0.704 | 0.688 |
| SUVpeak | 0.719 | 6.0 | 0.586 - 0.853 | 0.667 | 0.366 | |
| mSUVmtv5 | 0.694 | 6.0 | 0.547 - 0.841 | 0.667 | 0.680 | |
| mSUVmtv4.5 | 0.747 | 5.6 | 0.617 - 0.877 | 0.667 | 0.667 | |
| mSUVmtv4 | 0.741 | 5.3 | 0.612 - 0.870 | 0.667 | 0.677 | |
| mSUVmtv3.5 | 0.736 | 4.9 | 0.609 - 0.864 | 0.667 | 0.656 | |
| mSUVmtv3 | 0.722 | 4.5 | 0.591 - 0.852 | 0.667 | 0.656 | |
| mSUVmtv2.5 | 0.718 | 4.1 | 0.588 - 0.848 | 0.667 | 0.656 | |
| mSUVmtv2 | 0.668 | 3.7 | 0.531 - 0.806 | 0.593 | 0.594 | |
| Malignant/ | SUVmax | 0.770 | 7.6 | 0.668 - 0.872 | 0.686 | 0.688 |
| Premalignant | SUVpeak | 0.742 | 6.0 | 0.635 - 0.848 | 0.647 | 0.656 |
| mSUVmtv5 | 0.730 | 6.0 | 0.613 - 0.847 | 0.680 | 0.680 | |
| mSUVmtv4.5 | 0.761 | 5.6 | 0.656 - 0.867 | 0.667 | 0.667 | |
| mSUVmtv4 | 0.761 | 5.2 | 0.656 - 0.866 | 0.686 | 0.677 | |
| mSUVmtv3.5 | 0.763 | 4.9 | 0.658 - 0.867 | 0.667 | 0.656 | |
| mSUVmtv3 | 0.752 | 4.5 | 0.645 - 0.859 | 0.667 | 0.656 | |
| mSUVmtv2.5 | 0.745 | 4.1 | 0.636 - 0.854 | 0.647 | 0.656 | |
| mSUVmtv2 | 0.694 | 3.7 | 0.577 - 0.810 | 0.588 | 0.594 |
The long diameters of the malignant lesions were determined histopathologically after surgery, with an average of 32.8 ± 23.3 mm. Using the parametric method (Pearson correlation), SUVpeak was moderately positively correlated with tumor size, with a correlation coefficient (r) of 0.511. The mSUVmtv# (# = 2, 2.5, 3, 3.5, and 4) also showed moderate positive correlations. Using non-parametric methods, mSUVmtv# (# = 2, 2.5, and 3, Spearman's rho, r = 0.457 - 0.522) and mSUVmtv2 (Kendall's tau, r = 0.349) showed moderate or weak positive correlations.
Non-malignant intestinal FDG uptake occurs under several conditions, including inflammation[11-14] and the use of medications such as metformin[15-18]. This uptake may be diffuse, intense, and cover a large portion of the intestine. In such cases, it is not easy to identify obscured or hidden lesions. However, the presence of focal FDG uptake in the intestine suggests the need for further evaluation for malignant lesions.
The SUV is a representative semi-quantitative parameter for PET/CT. A high SUV could be more suggestive of malignancy than a benign lesion or physiologic uptake and might be associated with advanced disease or poor prognosis/overall survival in various cancers[19-25]. The present study assessed the clinical significance of incidental focal colorectal uptake by analyzing FDG PET parameters.
The detection rate of unexpected focal colorectal uptake in this study was 0.53% (80/15,143), consistent with the range of 0.5% - 3.3% reported by other studies[26-31]. A meta-analysis reported that a pooled prevalence of focal colorectal incidentalomas of 3.6%[32]. Of the 83 eligible lesions in this study, 51 (61.4%) were malignant (28.9%, 24/83) or premalignant (32.5%, 27/83). The remaining 32 (38.6%) were benign lesions or physiologic uptake. The proportion of premalignant lesions was slightly larger than that of malignant lesions, consistent with other studies[33,34]. The rate (61.4%, 51/83) of malignant/premalignant lesions was also comparable to that in other studies[32] and colonoscopy was recommended for further evaluation of focal hypermetabolism[35].
SUVmax, SUVpeak, and all mSUVmtv# differentiated malignant and premalignant lesions from benign lesions and physiologic uptake. According to the AUC curves, mSUVmtv3.5, with an AUC of 0.792 and a cut-off of 4.9, showed the best performance in distinguishing between malignant and benign lesions. Other mSUVmtv#s were also useful in identifying malignant lesions; however, as the # of mSUVmtv# approached extreme values (2 or 5, for instance), the boundaries of the visible MTV segmentations tended to be smaller or larger than the actual visible tumor boundaries. Thus, they might not have accurately reflected the MTV and, therefore, mSUVmtv. Practically, SUVmax, which is the most used among these parameters in the clinical setting, showed a similar AUC (0.784) and higher sensitivity and specificity, suggesting that it could replace mSUVmtv3.5. If the SUVmax is used as a determining factor, 7.6 would be the optimal cut-off. As shown in Table 3, the cut-offs for malignant lesions are similar to those for premalignant lesions, in which the malignant lesions are hardly distinguishable from premalignant lesions using the cut-offs derived in this study. None of the parameters involved in this study could distinguish them by statistical comparisons (P > 0.05). Other studies have shown inconsistent results[33,34], and some studies reported that even the SUVs of malignant lesions were not distinguishable from those of non-pathologic FDG uptake[27,28]. MTV and TLG were not useful for differentiating malignant and premalignant lesions from benign lesions. Both parameters showed better results than the SUVmax in other studies[36]. By combining malignant and premalignant lesions into one group, SUVmax (AUC 0.770, cut-off 7.6) was superior in distinguishing this group from benign focal colorectal hypermetabolism.
Among the 24 malignant lesions, regardless of the tumor type, 18 (75.0%) were located in the distal colon/rectum, and of the 27 premalignant lesions, 16 (59.3%) were in the proximal colon. Different genetic mechanisms play roles in cancer development in the distal or proximal colon[37-39] and different frequent locations were suggested in various studies[40-42]. Moreover, the distribution of colorectal cancer appears to vary by country, region, race, sex, and age[43-46]. Although the results of these studies are not always consistent, patient characteristics should be taken into account while interpreting PET/CT images. None of the parameters in this study differed significantly between the proximal and distal colon/rectum for malignant, premalignant, and malignant/premalignant lesions.
The long diameter of the malignant lesions was moderately to weakly positively correlated with several PET parameters (SUVpeak and a few mSUVmtv#); however, its clinical significance was unclear. In addition, SUVmax, which significantly distinguished malignant/premalignant from benign lesions, did not show any statistically significant correlations (P = 0.055).
This study was conducted retrospectively at a single institution. The incidental focal colorectal hypermetabolism discovered with the naked eye may have missed non/Less-FDG-avid pathologic lesions; therefore, there was a selection bias. For the same reason, the incidence of malignancy may be higher than that in the general population. As this study did not include focal hypermetabolism without histopathological reports, the results of this study might not be the same if there were pathological reports for all focal hypermetabolism. Despite these limitations, given the high frequency of malignant/premalignant lesions and statistically significant PET parameters, incidental focal colorectal FDG uptake has clinical significance; thus, the consideration of further assessment such as colonoscopy should not be avoided.
Approximately two-thirds (61.4%) of the incidentally observed focal hypermetabolic colorectal regions were malignant or premalignant. Although the role of FDG PET parameters in colorectal cancer remains controversial, the results of this study showed that SUVmax was an independent diagnostic parameter for malignant/premalignant lesions. Therefore, any unexpected suspicious focal colorectal FDG uptake requires attention, and further evaluation is strongly recommended not to miss the two-thirds.
Intestinal fluorine-18 fluorodeoxyglucose (F-18 FDG) uptake is often observed on positron emission tomography/computed tomography (PET/CT). However, unexpectedly observed focal colorectal hypermetabolism might harbor a risk of malignancy; thus, distinguishing malignant from benign tumors is critical.
As with other cancers, early lesion detection is critical in colorectal cancer. As surgery may still be the treatment of choice for cure in selected patients with advanced colorectal cancer, the importance of early detection of lesions is even greater.
To assess the implications of focal colorectal F-18 FDG uptake by analyzing FDG PET parameters.
This study included 83 focal colorectal hypermetabolic regions from 80 patients. Each region was classified as malignant, premalignant, or benign according to the histopathological report. PET parameters such as maximum and peak standardized uptake values (SUVmax and SUVpeak), metabolic tumor volume (MTV), mean SUV of metabolic tumor volume (mSUVmtv), and total lesion glycolysis (TLG) of F-18 FDG PET/CT were measured and calculated for the regions, and compared among malignant, premalignant, malignant/premalignant, and benign hypermetabolic regions. Receiver operating characteristic (ROC) curves were plotted to determine the cut-off values for these parameters.
Of the 83 incidentally observed focal colorectal hypermetabolic regions on F-18 FDG PET-CT, 61.4% (51/83) were malignant/premalignant lesions confirmed by histopathological reports of the corresponding locations. SUVmax, SUVpeak, and mSUVmtv can be used to differentiate malignant and premalignant lesions from benign lesions. SUVmax, with an AUC of 0.770 and a cut-off of 7.6 (confidence interval: 0.668–0.872, sensitivity 0.686, specificity 0.688) was the superior FDG PET parameter in distinguishing malignant and premalignant from benign lesions.
Approximately two-thirds (61.4%) of the incidental focal hypermetabolic colorectal regions were malignant/premalignant. SUVmax was demonstrated as an independent diagnostic parameter for the lesions. Unexpected suspicious focal colorectal FDG uptake should not be avoided and further evaluation is required.
Controversies and debates regarding the parameters assessed in this study remain ongoing. Further studies with larger numbers of subjects are warranted.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Z, China; Tsujinaka S, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 43146] [Article Influence: 14382.0] [Reference Citation Analysis (47)] |
| 3. | Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. Radiographics. 2007;27:145-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Ahmad Sarji S. Physiological uptake in FDG PET simulating disease. Biomed Imaging Interv J. 2006;2:e59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Zukotynski K, Kim CK. Abdomen: normal variations and benign conditions resulting in uptake on FDG-PET/CT. PET Clin. 2014;9:169-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Shammas A, Lim R, Charron M. Pediatric FDG PET/CT: physiologic uptake, normal variants, and benign conditions. Radiographics. 2009;29:1467-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Engel H, Steinert H, Buck A, Berthold T, Huch Böni RA, von Schulthess GK. Whole-body PET: physiological and artifactual fluorodeoxyglucose accumulations. J Nucl Med. 1996;37:441-446. [PubMed] [Cited in This Article: ] |
| 8. | Sone Y, Sobajima A, Kawachi T, Kohara S, Kato K, Naganawa S. Ability of 18-fludeoxyglucose positron emission tomography/CT to detect incidental cancer. Br J Radiol. 2014;87:20140030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Wu GZ, Sun D, Chen JY, Qiu JM, Kong Y. [Clinical diagnostic value of (18)F-FDG PET-CT in incidental finding of focal hypermetabolism focus in the colon and rectum]. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:555-560. [PubMed] [Cited in This Article: ] |
| 10. | Salazar Andía G, Prieto Soriano A, Ortega Candil A, Cabrera Martín MN, González Roiz C, Ortiz Zapata JJ, Cardona Arboniés J, Lapeña Gutiérrez L, Carreras Delgado JL. Clinical relevance of incidental finding of focal uptakes in the colon during 18F-FDG PET/CT studies in oncology patients without known colorectal carcinoma and evaluation of the impact on management. Rev Esp Med Nucl Imagen Mol. 2012;31:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Ahn BC, Lee SW, Lee J. Intense accumulation of F-18 FDG in colonic wall in adult onset still disease with pseudomembranous colitis. Clin Nucl Med. 2008;33:806-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Kjaer ASL, Ribberholt I, Thomsen K, Ibsen PH, Markova E, Graff J. 18F-FDG PET/CT Findings in Cytomegalovirus Colitis. Diagnostics (Basel). 2018;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Parbo P, Stribolt K, Rittig CS, Gormsen LC. Active ulcerative colitis diagnosed by (18)F-FDG PET/CT in an anti-TNF alpha treated patient with no visible luminal lesions on colonoscopy. Int J Colorectal Dis. 2014;29:643-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Hannah A, Scott AM, Akhurst T, Berlangieri S, Bishop J, McKay WJ. Abnormal colonic accumulation of fluorine-18-FDG in pseudomembranous colitis. J Nucl Med. 1996;37:1683-1685. [PubMed] [Cited in This Article: ] |
| 15. | Bevilacqua T, Greene GS. Diffuse bowel uptake of 18F-FDG on PET/CT examination of a patient with diabetes treated with metformin. BMJ Case Rep. 2014;2014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Bahler L, Stroek K, Hoekstra JB, Verberne HJ, Holleman F. Metformin-related colonic glucose uptake; potential role for increasing glucose disposal? Diabetes Res Clin Pract. 2016;114:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lee SH, Jin S, Lee HS, Ryu JS, Lee JJ. Metformin discontinuation less than 72 h is suboptimal for F-18 FDG PET/CT interpretation of the bowel. Ann Nucl Med. 2016;30:629-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Bybel B, Greenberg ID, Paterson J, Ducharme J, Leslie WD. Increased F-18 FDG intestinal uptake in diabetic patients on metformin: a matched case-control analysis. Clin Nucl Med. 2011;36:452-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Cheng NM, Hsieh CE, Liao CT, Ng SH, Wang HM, Fang YD, Chou WC, Lin CY, Yen TC. Prognostic Value of Tumor Heterogeneity and SUVmax of Pretreatment 18F-FDG PET/CT for Salivary Gland Carcinoma With High-Risk Histology. Clin Nucl Med. 2019;44:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Chin AL, Kumar KA, Guo HH, Maxim PG, Wakelee H, Neal JW, Diehn M, Loo BW Jr, Gensheimer MF. Prognostic Value of Pretreatment FDG-PET Parameters in High-dose Image-guided Radiotherapy for Oligometastatic Non-Small-cell Lung Cancer. Clin Lung Cancer. 2018;19:e581-e588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Kwon SY, Choi EK, Kong EJ, Chong A, Ha JM, Chun KA, Cho IH, Bom HS, Min JJ, Kim J, Song HC, O JH, Kim SH. Prognostic value of preoperative 18F-FDG PET/CT in papillary thyroid cancer patients with a high metastatic lymph node ratio: a multicenter retrospective cohort study. Nucl Med Commun. 2017;38:402-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Özgü E, Öz M, Yıldız Y, Özgü BS, Erkaya S, Güngör T. Prognostic value of 18F-FDG PET/CT for identifying high- and low-risk endometrial cancer patients. Ginekol Pol. 2016;87:493-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Bahri H, Laurence L, Edeline J, Leghzali H, Devillers A, Raoul JL, Cuggia M, Mesbah H, Clement B, Boucher E, Garin E. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med. 2014;55:1786-1790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Fuglø HM, Jørgensen SM, Loft A, Hovgaard D, Petersen MM. The diagnostic and prognostic value of ¹⁸F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur J Nucl Med Mol Imaging. 2012;39:1416-1424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 26. | Treglia G, Calcagni ML, Rufini V, Leccisotti L, Meduri GM, Spitilli MG, Dambra DP, De Gaetano AM, Giordano A. Clinical significance of incidental focal colorectal (18)F-fluorodeoxyglucose uptake: our experience and a review of the literature. Colorectal Dis. 2012;14:174-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Kei PL, Vikram R, Yeung HW, Stroehlein JR, Macapinlac HA. Incidental finding of focal FDG uptake in the bowel during PET/CT: CT features and correlation with histopathologic results. AJR Am J Roentgenol. 2010;194:W401-W406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Weston BR, Iyer RB, Qiao W, Lee JH, Bresalier RS, Ross WA. Ability of integrated positron emission and computed tomography to detect significant colonic pathology: the experience of a tertiary cancer center. Cancer. 2010;116:1454-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | van Hoeij FB, Keijsers RG, Loffeld BC, Dun G, Stadhouders PH, Weusten BL. Incidental colonic focal FDG uptake on PET/CT: can the maximum standardized uptake value (SUVmax) guide us in the timing of colonoscopy? Eur J Nucl Med Mol Imaging. 2015;42:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Peng J, He Y, Xu J, Sheng J, Cai S, Zhang Z. Detection of incidental colorectal tumours with 18F-labelled 2-fluoro-2-deoxyglucose positron emission tomography/computed tomography scans: results of a prospective study. Colorectal Dis. 2011;13:e374-e378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Oh JR, Min JJ, Song HC, Chong A, Kim GE, Choi C, Seo JH, Bom HS. A stepwise approach using metabolic volume and SUVmax to differentiate malignancy and dysplasia from benign colonic uptakes on 18F-FDG PET/CT. Clin Nucl Med. 2012;37:e134-e140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Treglia G, Taralli S, Salsano M, Muoio B, Sadeghi R, Giovanella L. Prevalence and malignancy risk of focal colorectal incidental uptake detected by (18)F-FDG-PET or PET/CT: a meta-analysis. Radiol Oncol. 2014;48:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Seivert M, Plomteux O, Colard A, Leclercq P, Gauthier D, Houbiers G, Dupont P, Demoulin JC, Fontaine F, Namur G, Witvrouw N, Bastens B. Endoscopic findings in case of incidental colonic uptake in PET-CT how to improve PET-CT specificity? Acta Gastroenterol Belg. 2014;77:413-417. [PubMed] [Cited in This Article: ] |
| 34. | Purandare NC, Gawade SK, Puranik AD, Agrawal A, Shah S, Rangarajan V. Etiology and significance of incidentally detected focal colonic uptake on FDG PET/CT. Indian J Radiol Imaging. 2012;22:260-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Roh SH, Jung SA, Kim SE, Kim HI, Lee MJ, Tae CH, Choi JY, Shim KN, Jung HK, Kim TH, Yoo K, Moon IH, Kim BS. The Clinical Meaning of Benign Colon Uptake in (18)F-FDG PET: Comparison with Colonoscopic Findings. Clin Endosc. 2012;45:145-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Murakami K, Kitagawa Y. Metabolic Tumor Volume and Total Lesion Glycolysis in PET/CT Correlate With the Pathological Findings of Colorectal Cancer and Allow Its Accurate Staging. Clin Nucl Med. 2016;41:761-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 526] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 38. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8087] [Cited by in F6Publishing: 7708] [Article Influence: 226.7] [Reference Citation Analysis (1)] |
| 39. | Beart RW, Melton LJ 3rd, Maruta M, Dockerty MB, Frydenberg HB, O'Fallon WM. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983;26:393-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2526] [Cited by in F6Publishing: 2757] [Article Influence: 393.9] [Reference Citation Analysis (2)] |
| 41. | Shmidt E, Nehra V, Lowe V, Oxentenko AS. Clinical significance of incidental [18 F]FDG uptake in the gastrointestinal tract on PET/CT imaging: a retrospective cohort study. BMC Gastroenterol. 2016;16:125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Golfam F, Golfam P, Neghabi Z. Frequency of all types of colorectal tumors in the patients referred to selected hospitals in tehran. Iran Red Crescent Med J. 2013;15:473-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Qing SH, Rao KY, Jiang HY, Wexner SD. Racial differences in the anatomical distribution of colorectal cancer: a study of differences between American and Chinese patients. World J Gastroenterol. 2003;9:721-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 44. | Thomas CR Jr, Jarosz R, Evans N. Racial differences in the anatomical distribution of colon cancer. Arch Surg. 1992;127:1241-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167-5175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 274] [Cited by in F6Publishing: 268] [Article Influence: 29.8] [Reference Citation Analysis (4)] |
| 46. | Schmuck R, Gerken M, Teegen EM, Krebs I, Klinkhammer-Schalke M, Aigner F, Pratschke J, Rau B, Benz S. Gender comparison of clinical, histopathological, therapeutic and outcome factors in 185,967 colon cancer patients. Langenbecks Arch Surg. 2020;405:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |