Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9825
Peer-review started: August 18, 2021
First decision: September 29, 2021
Revised: October 8, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: November 16, 2021
In malignant tumors, inflammation plays a vital role in the development, invasion, and metastasis of cancer cells. Diffuse large B-cell lymphoma (DLBCL), the most common malignant proliferative disease of the lymphatic system, is commonly associated with inflammation. The international prognostic index (IPI), which includes age, lactate dehydrogenase (LDH), number of extranodal lesions, Ann Arbor score, and Eastern Cooperative Oncology Group (ECOG) score, can evaluate the prognosis of DLBCL. However, its use in accurately identifying high-risk patients and guiding treatment is poor. Therefore, it is important to find novel immune markers in predicting the prognosis of DLBCL patients.
To determine the association between the systemic immune inflammation index (SII), ratio of lymphocytes to monocytes (LMR), ratio of LMR to LDH (LMR/LDH), and prognosis of patients with DLBCL.
A total of 68 patients diagnosed with DLBCL, treated in our hospital between January 2016 and January 2020, were included. χ2 test, Pearson’s R correlation, Kaplan Meier curves, and Cox proportional risk regression analysis were used. The differences in the SII, LMR, and LMR/LDH among patients with different clinicopathological features were analyzed. The differences in progression-free survival time among patients with different SII, LMR, and LMR/LDH expressions and influencing factors affecting the prognosis of DLBCL patients, were also analyzed.
The LMR and LMR/LDH in patients with Ann Arbor stage III–IV, ECOG score ≥ 2, and SII, IPI score 2–5 were significantly higher than those of patients with Ann Arbor stage I-II and ECOG score < 2 (P < 0.05). Patients with high SII, LMR, and LMR/LDH had progression-free survival times of 34 mo (95%CI: 32.52–38.50), 35 mo (95%CI: 33.42–36.58) and 35 mo (95%CI: 33.49–36.51), respectively, which were significantly lower than those with low SII, LMR, and LMR/LDH (P < 0.05); the SII, LMR, and LMR/LDH were positively correlated (P < 0.05). Cox proportional risk regression analysis showed that the SII, LMR, and LMR/LDH were influencing factors for the prognosis of DLBCL patients (hazard ratio = 1.143, 1.665, and 1.704, respectively; P < 0.05).
The SII, LMR, and LMR/LDH are related to the clinicopathological features of DLCBL, and they also influence the prognosis of patients with the disease.
Core Tip: We need find effective biomarkers to predict the prognosis and recurrence of diffuse large B-cell lymphoma among patients. Lactate dehydrogenase and systemic immune inflammation index can be used as a prognostic indicator.
- Citation: Wu XB, Hou SL, Liu H. Systemic immune inflammation index, ratio of lymphocytes to monocytes, lactate dehydrogenase and prognosis of diffuse large B-cell lymphoma patients. World J Clin Cases 2021; 9(32): 9825-9834
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9825.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9825
Diffuse large B-cell lymphoma (DLBCL), the most common type of non-Hodgkin lymphoma (NHL), is the result of the combined effect of immune stimulation, immunosuppression, and genetic susceptibility of patients[1,2]. Currently, 75%-80% of DLBCL patients can achieve a complete response after first-line treatment. However, studies have suggested that approximately 40% of patients still experience relapse and/or resistance to treatment owing to the highly heterogeneous nature of the disease, especially in terms of cell origin, biological features, clinical manifestations, prognosis, and other aspects, such as its tendency to be highly invasive[3]. Therefore, finding effective biomarkers to predict the prognosis and recurrence of DLBCL among patients is still needed. Studies show that the ratio of lymphocytes to monocytes (LMR) can best reflect the host immune status; thus, it can be used as an effective prognostic marker in DLBCL patients[4]. In addition, the ratio of immune cells to tumor load can be used as a prognostic indicator for these patients. Studies have also shown that in lymphoma, lactate dehydrogenase (LDH) is associated with the tumor microenvironment and tumor tissue DNA, which is an effective marker reflecting tumor load[5]. The systemic immune inflammation index (SII) is a comprehensive inflammatory marker based on peripheral blood neutrophil, platelet, and lymphocyte counts, which can be used to predict the clinical prognosis of non-small cell lung cancer, gastric cancer, pancreatic cancer, and many other solid tumors[6,7]; however, there are still few studies on its use in DLBCL. Our study mainly explored and discussed the association between the SII, LMR, LMR/LDH, and prognosis of DLBCL patients.
A total of 68 DLBCL patients treated in our hospital between January 2016 and January 2020 were selected. Of these, 40 were male, and 28 were female. There were 22 patients aged < 60 years, and 46 aged > 60 years. There were 41 patients with Ann Arbor stage I–II and 27 with stage III–IV. A total of 46 patients had an Eastern Cooperative Oncology Group (ECOG) score < 2, while 22 had an ECOG score ≥ 2. Thirty-six patients had an international prognostic index (IPI) score of 0–1, and 32 had an IPI score of 2–5. The inclusion criteria were as follows: (1) the diagnosis meets the criteria in the Chinese Guidelines for the Diagnosis and Treatment of DLBCL[8]; (2) primary treatment; (3) receiving R-CHOP or R-CHOP-like chemotherapy regimens in our hospital; (4) clinical follow-up data kept intact; and (5) informed consent of patients and their families. The exclusion criteria were as follows: (1) complicated disease with systemic malignancies; (2) complicated with heart, liver, kidney, and other organ diseases; and (3) giving up treatment halfway.
All patients received standard immunochemotherapy, which included rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (D1: cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 and D1–5: prednisone 100 mg/m2 and rituximab 375 mg/m2 for each cycle). Each cycle of treatment was administered with an interval of 21 d for least six cycles. All patients were followed up regularly. Follow-up included the patient's current health state, assessment for recurrence, and the time and cause of death if the patient died. The follow-up date was up to May 31, 2020.
Factors that may be related to the prognosis of DLBCL patients were included in the study, such as sex, age, pathological type, clinical stage, IPI score, ECOG score, LDH, absolute value of lymphocytes (ALC), absolute value of monocytes (AMC), LMR (ALC/AMC), and SII (%) [(neutrophil count × platelet count)/Lymphocyte count × 100%].
SPSS 22.0 software was used for analysis, measurement data were expressed as mean ± SD, and t test was used for comparison between groups. Enumeration data were expressed as n (%), and comparison between groups was performed using χ2 test. Pearson correlation analysis was used for correlation analysis. The survival curve was analyzed using the Kaplan–Meier method. Prognostic factors were analyzed using the Cox proportional risk regression (inspection a = 0.05).
The SII in patients with Ann Arbor stage III–IV and ECOG score ≥ 2 was significantly higher than that of patients with Ann Arbor stage I–II and ECOG score < 2 (P < 0.05), as shown in Table 1.
| Clinicopathological features | n | SII (%) | t | P value |
| Sex | -0.321 | 0.749 | ||
| Male | 40 | 519.29 ± 98.29 | ||
| Female | 28 | 526.84 ± 91.10 | ||
| Age (yr) | -0.137 | 0.891 | ||
| < 60 | 22 | 520.02 ± 94.50 | ||
| ≥ 60 | 46 | 523.54 ± 101.10 | ||
| Ann Arbor stage | -2.377 | 0.020 | ||
| I-II | 41 | 501.22 ± 85.50 | ||
| III-IV | 27 | 554.56 ± 97.83 | ||
| ECOG scores | -2.906 | 0.005 | ||
| < 2 | 46 | 498.82 ± 95.30 | ||
| ≥ 2 | 22 | 571.70 ± 99.82 | ||
| IPI scores | -1.378 | 0.173 | ||
| 0-1 | 36 | 506.60 ± 101.02 | ||
| 2-5 | 32 | 540.07 ± 98.82 | ||
| Hans | -0.445 | 0.658 | ||
| GCB | 26 | 515.58 ± 100.43 | ||
| Non GCB | 42 | 526.62 ± 98.85 | ||
| BCL2 expression | 0.790 | 0.432 | ||
| < 50% | 40 | 530.03 ± 93.04 | ||
| ≥ 50% | 28 | 511.50 ± 98.20 | ||
| Ki-67 | -0.402 | 0.689 | ||
| < 70% | 23 | 515.53 ± 99.40 | ||
| ≥ 70% | 45 | 525.91 ± 101.43 |
The LMR in patients with Ann Arbor stage III–IV, ECOG score ≥ 2, and IPI score 2–5 was significantly higher than that of patients with Ann Arbor stage I–II, ECOG score < 2, and IPI score 0–1 (P < 0.05), as shown in Table 2. The LMR/LDH in patients with Ann Arbor stage III–IV, ECOG score ≥ 2, and IPI score 2–5 was significantly higher than that of patients with Ann Arbor stage I–II, ECOG score < 2, and IPI score 0–1 (P < 0.05), as shown in Table 3.
| Clinicopathological features | LMR | t | P value |
| Sex | -0.976 | 0.333 | |
| Male | 2.61 ± 0.45 | ||
| Female | 2.74 ± 0.65 | ||
| Age (yr) | 0.283 | 0.778 | |
| < 60 year | 2.70 ± 0.49 | ||
| ≥ 60 year | 2.66 ± 0.57 | ||
| Ann Arbor stage | -4.559 | < 0.001 | |
| I-II | 2.41 ± 0.52 | ||
| III-IV | 3.06 ± 0.64 | ||
| ECOG scores | -3.280 | 0.002 | |
| < 2 | 2.52 ± 0.50 | ||
| ≥ 2 | 2.98 ± 0.62 | ||
| IPI scores | -2.339 | 0.022 | |
| 0-1 | 2.54 ± 0.52 | ||
| 2-5 | 2.82 ± 0.46 | ||
| Hans | -0.338 | 0.737 | |
| GCB | 2.64 ± 0.43 | ||
| Non GCB | 2.68 ± 0.50 | ||
| BCL2 expression | -0.173 | 0.863 | |
| < 50% | 2.66 ± 0.44 | ||
| ≥ 50% | 2.68 ± 0.51 | ||
| Ki-67 | 0.442 | 0.660 | |
| < 70% | 2.71 ± 0.55 | ||
| ≥ 70% | 2.65 ± 0.52 |
| Clinicopathological features | LMR/LDH | t | P value |
| Sex | -1.947 | 0.056 | |
| Male | 0.38 ± 0.10 | ||
| Female | 0.43 ± 0.11 | ||
| Age (yr) | 0.796 | 0.429 | |
| < 60 | 0.41 ± 0.09 | ||
| ≥ 60 | 0.39 ± 0.10 | ||
| Ann Arbor stage | -4.799 | < 0.001 | |
| I-II | 0.36 ± 0.08 | ||
| III-IV | 0.46 ± 0.09 | ||
| ECOG scores | -5.977 | < .001 | |
| < 2 | 0.35 ± 0.09 | ||
| ≥ 2 | 0.50 ± 0.11 | ||
| IPI scores | -4.366 | < 0.001 | |
| 0-1 | 0.36 ± 0.08 | ||
| 2-5 | 0.45 ± 0.09 | ||
| Hans | 0.853 | 0.397 | |
| GCB | 0.41 ± 0.10 | ||
| Non GCB | 0.39 ± 0.09 | ||
| BCL2 expression | -0.964 | 0.339 | |
| < 50% | 0.39 ± 0.08 | ||
| ≥ 50% | 0.41 ± 0.09 | ||
| Ki-67 | 0.835 | 0.407 | |
| < 70% | 0.41 ± 0.10 | ||
| ≥ 70% | 0.39 ± 0.09 |
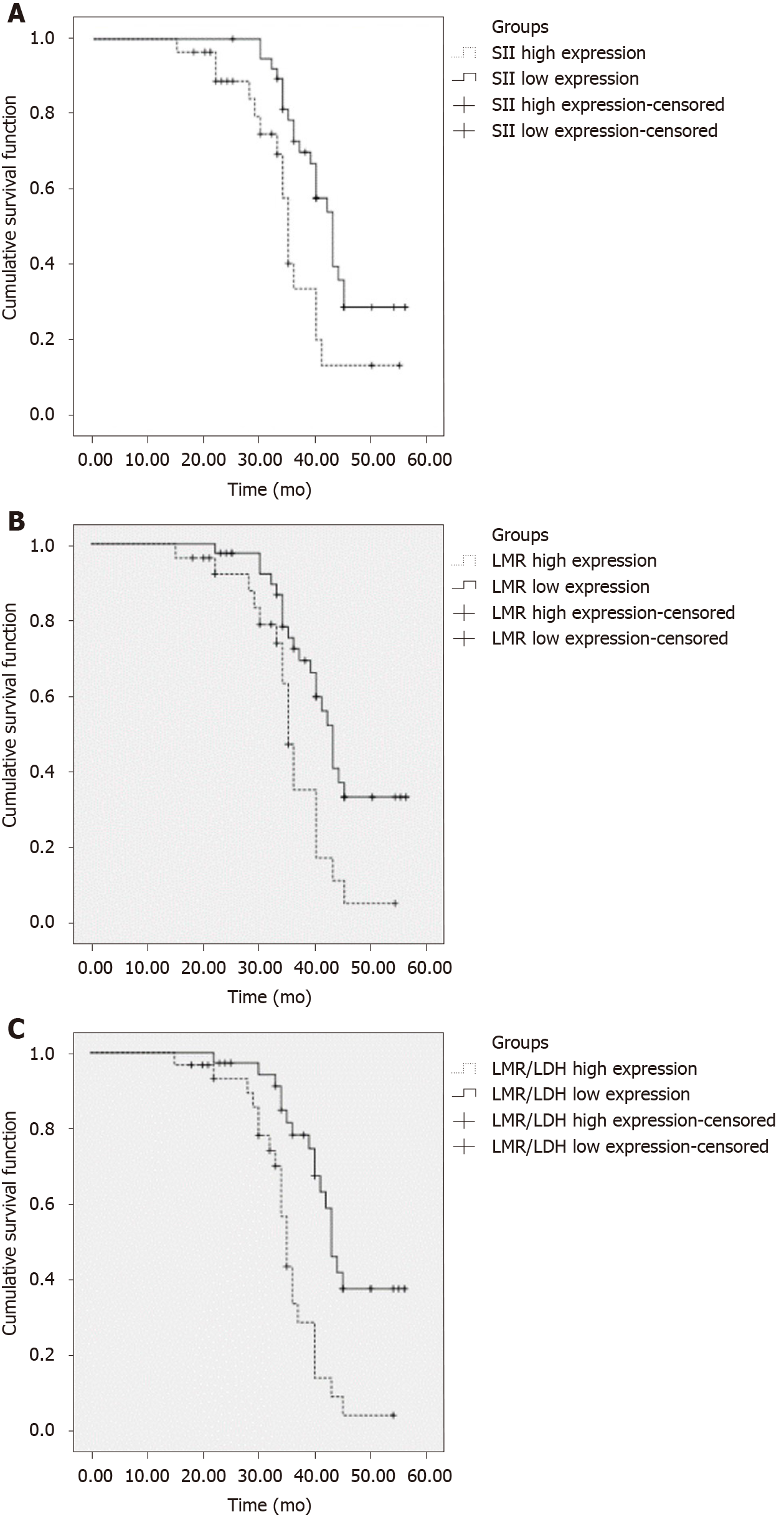
The progression-free survival times of patients with high SII (≥ 521.50), high LMR (≥ 2.67), and high LMR/LDH (> 0.40) were 34 mo (95%CI: 32.52–38.50), 35 mo (95%CI: 33.42–36.58), and 35 mo (95%CI: 33.49–36.5), respectively. These were significantly lower than those with low SII (< 521.50), low LMR (< 2.67), and low LMR/LDH (P < 0.05), as shown in Figure 1 and Table 4.
| Groups | Median progression free survival time | 95%CI | χ2 | P value |
| SII high expression | 34 mo | 32.52-38.50 | 7.742 | 0.222 |
| SII low expression | 42 mo | 39.50-46.16 | ||
| LMR high expression | 35 mo | 33.42-36.58 | 8.356 | 0.004 |
| LMR low expression | 43 mo | 40.64-45.36 | ||
| LMR/LDH high expression | 35 mo | 33.49-36.51 | 15.163 | 0.000 |
| LMR/LDH low expression | 43 mo | 40.73-45.27 |
Table 5 shows a significant positive correlation between the SII, LMR, and LMR/LDH (P < 0.05).
Clinicopathological features, the SII, the LMR, and the LMR/LDH were taken as independent variables and prognosis as a dependent variable for Cox proportional risk regression analysis. The results showed that the SII, LMR and LMR/LDH were influencing factors for the prognosis of DLBCL patients [hazard ratio (HR) = 1.143, 1.665, and 1.704, respectively; P < 0.05], as shown in Table 6.
| Index | HR (95%CI) | P value |
| Sex | 0.882 (0.810-1.102) | 0.443 |
| Age | 1.343 (1.102-1.928) | 0.021 |
| Ann Arbor stage | 1.892 (1.303-2.811) | 0.004 |
| ECOG scores | 1.783 (1.232-2.704) | 0.014 |
| IPI scores | 1.903 (1.454-2.554) | 0.009 |
| Hans | 0.928 (0.783-1.432) | 0.323 |
| BCL2 expression | 0.782 (0.662-1.044) | 0.314 |
| Ki-67 | 1.102 (0.922-1.782) | 0.401 |
| SII | 1.143 (1.044-1.604) | 0.020 |
| LMR | 1.665 (1.182-2.433) | 0.006 |
| LMR/LDH | 1.704 (1.115-2.302) | 0.004 |
In malignant tumors, inflammatory reaction plays a vital role in the progression, invasion, and metastasis of cancer cells. The most common malignant tumors of the hematological system are lymphomas. Lymphomas originate from lymph nodes and lymphoid tissue in the immune system. DLBCL is the most common subtype of lymphomas accounting for 30%–40% of all adult NHLs, and its occurrence is associated with inflammation. The IPI, which includes age, LDH, number of extranodal lesions, Ann Arbor score, and ECOG score, can evaluate the prognosis of DLBCL patients. However, its use in accurately identifying high-risk patients and guiding treatment decisions is poor. Therefore, finding other effective biomarkers to predict the prognosis of DLBCL patients is important. Studies have shown that inflammation has a dual function on malignant tumors[9-11]. Recently, some serum markers capable of measuring inflammatory states have been proposed. These markers, including the SII, LMR, and LMR/LDH, such as those found in this study, can predict the prognosis of patients with tumors[12].
Liu et al[13] suggested that the body’s immune reaction to tumor cells, tumor load, and microenvironment greatly affects the prognosis of DLBCL patients. Lymphocytes play an immune surveillance role, which can effectively remove the malignant cells in a normal patient. When the lymphocyte count decreases, it leads to insufficient tumor inhibition in the body and promotes tumor growth and metastasis[14]. A report showed that lymphomas have high morbidity in immunodeficient patients, which further illustrates the importance of immunosuppression in lymphoma proliferation[15]. Another Study has also suggested that the LMR can reflect the body’s immune status, and its decrease indicates host immune dysfunction. Thus, it can be used to predict the survival of patients with various malignant tumors[16].
DLBCL is a systemic disease that develops rapidly and often involves lymph nodes and other organs, such as the stomach, testes, and central nervous system[17]. Computed tomography (CT) is usually used to evaluate tumor size to determine tumor load. A positron emission tomography (PET)/CT scan is the most sensitive and has a high specificity, although due to its high cost, clinical application is usually limited. Some studies have shown that in DLBCL patients, PET/CT scan results were significantly positively correlated with serum LDH levels; thus, the LMR/LDH can be used to predict the prognosis of patients. The SII is a novel index, based on neutrophil, lymphocyte, and platelet counts, used as a composite indicator of the body’s inflammatory response. As previously discussed, inflammation is an independent risk factor of various malignant tumors, especially in post-treatment patients[18]. According to our study, the SII of patients with Ann Arbor stage III–IV and ECOG score ≥ 2 was significantly higher than that of those with Ann Arbor stage I–II and ECOG score < 2 (P < 0.05), indicating that the SII was closely related to the clinical stage and severity of disease. The ECOG score is the prognostic system scoring criteria for diffuse large B-cell lymphoma: the higher the score, the higher the Ann Arbor stage, the worse the patient's prognosis, and the more serious the disease progression. In our study, the progression-free survival time of patients with high SII (≥ 521.50) was significantly lower than that of those with low SII (< 521.50) (P < 0.05), indicating that the SII is related to the prognosis of DLBCL patients, consistent with previous studies. In addition, through multivariate analysis, our study also found that the SII is a significant factor influencing the prognosis of DLBCL patients (HR = 1.143, 1.665, and 1.704, respectively; P < 0.05).
In our study, the ratio of LMR to LDH was used as a marker of immune response and tumor load on the prognosis of DLBCL patients. Results have shown that, the progression-free survival time of patients with high LMR (≥ 2.67) and high LMR/LDH (> 0.40) was significantly shorter than that of those with low LMR (< 2.67) and low LMR/LDH (P < 0.05). These results suggest that the LMR/LDH and LMR reflect the immune status of the host, which is related to the prognosis of patients. Correlation analysis of the SII, LMR, and LMR/LDH showed that there was a positive correlation among the SII, LMR, and LMR/LDH (P < 0.05) and that the LMR and LMR/LDH were influencing factors for the prognosis of DLBCL patients (P < 0.05). Studies have found that the LMR is the independent index to monitor the recurrence and progression in DLBCL patients and that a decrease in the LMR is one of the factors in DLBCL recurrence[19,20]. Our study analyzed the effect of the LMR on the prognosis of DLBCL patients, which was consistent with the results of previous studies. Currently, there are few reports on the effect of the LMR/LDH on the prognosis of DLBCL patients.
Due to the small sample size, the results in this study may not be representative enough of DLBCL patients and thus may be prone to bias. Therefore, it is necessary to perform larger studies, such as a multicenter, large sample prospective study, on the relationship between cytokine levels and prognosis of DLBCL patients to help clinicians choose a reasonable therapeutic regimen.
In conclusion, the SII, LMR, and LMR/LDH in DLBCL patients were related to the clinicopathological features of the patients to a certain extent and were also influencing factors for the prognosis of DLBCL patients.
Diffuse large B-cell lymphoma (DLBCL), the most common malignant proliferative disease of the lymphatic system, is commonly associated with inflammation.
It is still necessary to find effective biomarkers to predict the prognosis and recurrence of DLBCL among patients.
We discussed the association between the systemic immune inflammation index (SII), ratio of lymphocytes to monocytes (LMR), ratio of LMR to lactate dehydrogenase (LDH), and prognosis of DLBCL patients.
Total 68 patients with diffuse large B-cell lymphoma selected for treatment in our hospital. The differences in the SII, LMR, and LMR/LDH among patients with different clinicopathological features were analyzed.
SII, LMR, and LMR/LDH were influencing factors for the prognosis of DLBCL patients.
The SII, LMR, and LMR/LDH in DLBCL patients were related to the clinicopathological features of the patients to a certain extent and were also influencing factors for the prognosis of DLBCL patients.
It is necessary to perform larger studies to help clinicians choose a reasonable therapeutic regimen.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adams HJA, Kempf W S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Abadi U, Peled L, Gurion R, Rotman-Pikielny P, Raanani P, Ellis MH, Rozovski U. Prevalence and clinical significance of hypercalcemia at diagnosis in diffuse large B-cell lymphoma. Leuk Lymphoma. 2019;60:2922-2926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Nowakowski GS, Feldman T, Rimsza LM, Westin JR, Witzig TE, Zinzani PL. Integrating precision medicine through evaluation of cell of origin in treatment planning for diffuse large B-cell lymphoma. Blood Cancer J. 2019;9:48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Matsuki E, Bohn OL, El Jamal S, Pichardo JD, Zelenetz AD, Younes A, Teruya-Feldstein J. Lymphocyte-to-Monocyte Ratio May Serve as a Better Prognostic Indicator Than Tumor-associated Macrophages in DLBCL Treated With Rituximab. Appl Immunohistochem Mol Morphol. 2019;27:572-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Gorodetskiy VR, Probatova NA, Radenska-Lopovok SG, Ryzhikova NV, Sidorova YV, Sudarikov AB. Clonal relationship of marginal zone lymphoma and diffuse large B-cell lymphoma in Sjogren's syndrome patients: case series study and review of the literature. Rheumatol Int. 2020;40:499-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol. 2018;19:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore). 2017;96:e5886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Lolli C, Basso U, Derosa L, Scarpi E, Sava T, Santoni M, Crabb SJ, Massari F, Aieta M, Conteduca V, Maruzzo M, La Russa F, Wheater M, Berardi R, Galli L, De Giorgi U. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016;7:54564-54571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 8. | Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50:74-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 9. | Chiappella A, Castellino A, Nicolosi M, Santambrogio E, Vitolo U. Diffuse Large B-cell Lymphoma in the elderly: standard treatment and new perspectives. Expert Rev Hematol. 2017;10:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 923] [Cited by in F6Publishing: 984] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 11. | Jomrich G, Paireder M, Kristo I, Baierl A, Ilhan-Mutlu A, Preusser M, Asari R, Schoppmann SF. High Systemic Immune-Inflammation Index is an Adverse Prognostic Factor for Patients With Gastroesophageal Adenocarcinoma. Ann Surg. 2021;273:532-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 12. | Kumarasamy C, Sabarimurugan S, Madurantakam RM, Lakhotiya K, Samiappan S, Baxi S, Nachimuthu R, Gothandam KM, Jayaraj R. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e14834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Liu J, Gao Y, Kan BH, Zhou L. [Systematic review and meta-analysis of randomized controlled trials of Chinese herbal medicine in treatment of multiple sclerosis]. Zhong Xi Yi Jie He Xue Bao. 2012;10:141-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yang Z, Yu W, Wang S, Zhou X, Liu S, Ma S. Peripheral blood lymphocyte subsets of newly diagnosed DLBCL patients and their dynamic changes with rituximab based immunochemotherapy. Leuk Lymphoma. 2019;60:2909-2916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Hu R, Winter A, Hill BT. The Emerging Role of Minimal Residual Disease Testing in Diffuse Large B-Cell Lymphoma. Curr Oncol Rep. 2019;21:44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Wang J, Gao K, Lei W, Dong L, Xuan Q, Feng M, Wang J, Ye X, Jin T, Zhang Z, Zhang Q. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget. 2017;8:5414-5425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Miyahara Y, Takashi S, Shimizu Y, Ohtsuka M. The prognostic impact of neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in patients with distal bile duct cancer. World J Surg Oncol. 2020;18:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Zhang SN, Li MJ, Yuan FF, Chen L, Mi RH, Wei XD, Song YP, Yin QS. [Efficacy and prognosis of the dynamic monitoring lymphocyte to monocyte ratio in patients with diffuse large B-cell lymphoma]. Zhonghua Yi Xue Za Zhi. 2019;99:3139-3144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Yan-Li L, Kang-Sheng G, Yue-Yin P, Yang J, Zhi-Min Z. The lower peripheral blood lymphocyte/monocyte ratio assessed during routine follow-up after standard first-line chemotherapy is a risk factor for predicting relapse in patients with diffuse large B-cell lymphoma. Leuk Res. 2014;38:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Yang D, Su LP. [Influence of Peripheral Blood Lymphocyte/Monocyte ratio (LMR) and Its Ratio to Lactate Dehydrogenase on Prognosis of Patients with Diffuse Large B-Cell Lymphoma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:1563-1569. [PubMed] [Cited in This Article: ] |