Published online Sep 16, 2014. doi: 10.12998/wjcc.v2.i9.402
Revised: June 17, 2014
Accepted: July 12, 2014
Published online: September 16, 2014
Interspinous posterior device (IPD) is a term used to identify a relatively recent group of implants used to treat lumbar spinal degenerative disease. This kind of device is classified as part of the group of the dynamic stabilization systems of the spine. The concept of dynamic stabilization has been replaced by that of dynamic neutralization of hypermobility, with the intention of clarifying that the primary aim of this kind of system is not the preservation of the movement, but the dynamic neutralization of the segmental hypermobility which is at the root of the pathological condition. The indications for the implantation of an IPD are spinal stenosis and neurogenic claudication, assuming that its function is the enlargement of the neural foramen and the decompression of the roots forming the cauda equina in the central part of the vertebral canal. In the last 10 years, use of these implants has been very common but to date, no long-term clinical follow-up regarding clinical and radiological aspects are available. The high rate of reoperation, recurrence of symptoms and progression of degenerative changes is evident in the literature. If these devices are effectively a miracle cure for lumbar spinal stenosis, why do the utilization and implantation of IPD remain extremely controversial and should they be investigated further? Excluding the problems related to the high cost of the device, the main problem remains the pathological substrate on which the device is explicit in its action: the degenerative pathology of the spine.
Core tip: If interspinous posterior devices are effectively a miracle cure for lumbar spinal stenosis, why does their use and implantation remain extremely controversial and should they be investigated further? The aim of this editorial is to analyze and underline why these devices have poor outcomes, focusing on a biomechanical point of view, trying to define indications and limits. Is important to underline that these implants must not become a trend but only a weapon in the surgeon’s hands and, as with every weapon, is extremely dangerous in the wrong hands. So the spinal surgeon is the only one who can decide when to use it and must know the effects of this weapon in detail to use it correctly with no damage for the patient.
- Citation: Landi A. Interspinous posterior devices: What is the real surgical indication? World J Clin Cases 2014; 2(9): 402-408
- URL: https://www.wjgnet.com/2307-8960/full/v2/i9/402.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i9.402
Interspinous posterior device (IPD) is a term used to identify a relatively recent group of implants used to treat lumbar spinal degenerative disease. This kind of device is classified as part of the group of the dynamic stabilization systems of the spine. The concept of dynamic stabilization has been replaced by that of dynamic neutralization of hypermobility, with the intention of clarifying that the primary aim of this kind of system is not the preservation of the movement, but the dynamic neutralization of the segmental hypermobility which is at the root of the pathological condition[1-9].
The indications for the implantation of an IPD are spinal stenosis and neurogenic claudication, assuming that its function is the enlargement of the neural foramen and the decompression of the roots forming the cauda equina in the central part of the vertebral canal.
IPDs have evolved, being classified into not restricted and restricted, based on the presence or the absence of a dynamic control of movements only in extension or flexion and extension respectively[10-27].
A further evolution has brought the development of the interspinous fusion device (IFD), another group of implants, whose aim is interspinous bone fusion. The aim of these devices is not the dynamic neutralization of the hypermovement, but bone fusion with a complete block of the metameric movement. In light of this, in my opinion, IFDs cannot be classified as movement dynamic control systems because their aim is the osseous fusion of the segment, so are completely different from IPD[16-21].
In the last 10 years, use of these implants has been very common but to date, no long-term clinical follow-up are available regarding clinical and radiological aspects. The high rate of reoperation, recurrence of symptoms and progression of degenerative changes is evident in the literature. But the real question is this: If these devices are effectively a miracle cure for lumbar spinal stenosis, why does the utilization and implantation of IPD remain extremely controversial and should they be investigated further? Excluding the problems related to the high cost of the device, the main problem remains the pathological substrate on which the device is explicit in its action: the degenerative pathology of the spine.
If we consider that IPD can be implanted in stenosis of a mild and moderate degree in central or foraminal stenosis, or in low grade spondylolisthesis without spondylolysis (with poor or at least controversial results), we take for granted that the degenerative lumbar cascade, as described by Kirkaldy-Willis, is in the active phase[22-27].
Degenerative lumbar spondylosis in the active phase as a first step has the damage of the intervertebral disc, whose degree of degeneration is related to the entity of the damage and the persistency of the damage itself in time[22-27].
Normally, the biomechanical behavior of the lumbar spine is subject to the rule of spine loading. According to this rule, the axial load of the body is discharged and consequently neutralized on the intervertebral disc and the posterior structures (articulations, ligaments and muscles) in proportions of 80% and 20% respectively[27].
Any disc degeneration transfers the axial load to the posterior elements of the spine, determining an inversion in the distribution of the axial load related to the loss of viscoelastic and shock absorber properties of the disc itself. This condition promotes the insurgence of a functional overload of the facet joints, determining a greater mechanical stress than the physiological one, with consequent hyperlaxity of the facet joints, reduced competence of the articular capsule and hypermobility of the lumbar segment[22-27].
The hypermobility stimulates the inflammatory reaction in the adjacent tissues, activating chemokines (fractalkine in particular) in the ligamentum flavum, promoting chemotaxis in the ligamentum itself. The inflammatory cells cause extracellular matrix degradation of the ligamentum, determining loss of elasticity and hypertrophy. The role of fractalkine is well documented in the development of numerous inflammatory diseases (rheumatoid arthritis, dermatitis, etc.) and in ligaments and joints involved in inflammatory processes caused by instability (e.g., joint capsules, ligaments and synovium). The inflammatory process involves these tissues so the fractalkine overexpression is activated, thus causing the recruitment of mononuclear cells within the LF, feeding the inflammation and causing vascular injury and angiogenesis[20]. Moreover, the increase in mononuclear activity causes a proliferation of fibroblasts (for overexpression of TGF beta mRNA resulting in increased collagen fibers) and inflammatory cells in LF. This inflammatory cell activity in the LF causes rupture of the extracellular matrix (for activation of metalloproteinase MMP2) due to the elastin degradation, resulting in loss of elasticity of the ligament and subsequent hypertrophy[22-27].
The collapse of the intervertebral disc causes ligamentum flavum redundancy and its prominence in the vertebral canal reduces the diameter of the canal itself, determining spinal stenosis.
Only in this phase, the articular hypertrophy generates foraminal stenosis, the collapse of the disc generates ligamentous stenosis and the stenosis becomes symptomatic, but the main pathological substrate remains the hypermobility[22-27]. The treatment of a hard or soft stenosis has to be strictly linked to the concept of vertebral instability as a basic pathological condition. Relating to this concept, the commercialized IPDs have many biomechanical weaknesses that, in my opinion, should make their use extremely rare if not contraindicated.
Non-restricted IPD is a heterogeneous and very populous group of implants (X-STOP, Aperius, Bacjac, Ellipse etc). When implanted, their main aim is the interspinous posterior distraction to open the intervertebral foramina. Their primary effect is the decompression of nerve roots in their passage through the foramina. From a biomechanical point of view, the implant of this device has consequences on the involved and adjacent segments: (1) The axial load is shifted anteriorly on a degenerated intervertebral disc in which degeneration promotes the lumbar stenosis. The anterior load statically and dynamically over-solicits a degenerated disc, which has partially lost its features of shock absorber and elastic resistance against movements, promoting a faster degeneration of the disc; and (2) The distraction needed to open the intervertebral foramina causes an alteration of the lumbar spine sagittal balance[28-42].
Sagittal balance is the axial equilibrium that the whole spine has towards the outside world; its integrity provides elastic properties to the spine and tolerance to loads. Sagittal balance is based mainly on an adequate equilibrium of the physiological curvatures of the spine so that they can transfer the axial load to the floor, passing through the hips and the heads of the femora. The load line of the axial load is a vertical vector perpendicular to the floor passing through the external acoustic meatus, the midpoint of the endplate of L5 and the head of the femur. This vector has to always be posterior to the line connecting the two heads of the femora: to achieve this aim, the spine curvatures have to be maintained physiologically as much as possible. In particular, the preservation of physiological lumbar lordosis is fundamental[36-42].
The purpose of these devices is their implant between the spinous processes and their distraction; this movement of distraction transfers the axial load in the anterior compartment on a degenerated disc and alters the biomechanics of the whole spine, with a negative impact on the sagittal balance. This action has consequences on the spine, determining postural alterations, rotations of the spino-pelvic alignment and alterations in the thoracic and cervical curvatures, trying to compensate the alteration of the sagittal balance but actually accelerating the progression of the spinal degeneration. These patients are in a condition of spinal imbalance[36-42].
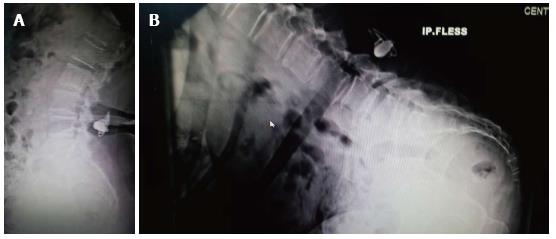
Initially, patients can have an improvement of their symptoms due to the foraminal decompression but long-term the alteration of the spinal biomechanics can only accelerate the degenerative process, with involvement of the treated and adjacent segments (Figure 1).
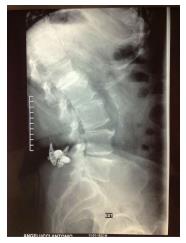
Furthermore, the overload applied to the spinous processes can cause a fracture of the processes themselves or lacerations of the posterior longitudinal ligament, causing the mobilization of the device (Figure 2)[36-49] .
The restricted IPDs (such as Wallis, Diam, Intraspine, etc.) have the presumed function of neutralizing the movements of flexion and extension at excessive degrees. These implants have the distraction of the spinous processes to widen the intervertebral foramina as a fundamental step, altering the biomechanics of the lumbar spine and determining sagittal imbalance, with the same mechanism as the non restricted IPD. Although these devices can control the excessive degrees of movements in flexion and extension, they cause a non physiological alteration in the movements of the spinal motor unit, with the same consequences described before. Furthermore, the segmental instability is not limited to the simple movements of flexion and extension, but also the movements of lateral bending and axial rotation, often associated with the movements of flexion and extension while the spine executes complex movements.
An interspinous device cannot control the movements of axial rotation and lateral bending, highly solicited after the implant of the device, accelerating and fastening the degenerative process.
IPDs are defined as movement preserving devices, but they are not explicit in this action for many reasons: (1) Their implantation puts the lumbar spine in a kyphotic posture so that it cannot move in a physiological way. In light of this, the movement cannot be intended as preserved; (2) The movement of the spinal motor unit depends greatly on the articular masses, the inter- and supraspinous ligament and the muscles of the posterior tension band, and all the components of the spinal motor unit tend to degeneration; for these reasons, all IPDs are not capable of controlling the movements in all three directions of the space and substituting all the components of the motor unit, so they cannot be defined as dynamic stabilization[28-35,43-46,50]; and (3) The materials and biomechanical concepts of construction of these devices are not fully respectful of the biological characteristics of human tissues[28-35,43-46,50-63].
Different considerations have to be made for interspinous fusion devices (IFD) (Aspen, Axle, etc). Described in the past, this technique has been brought to the fore in the last few years with the development of new spinous-anchoring devices whose aim is an interspinous bone fusion.
The main goal of IPD is motion preservation, while IFDs have a different root concept: if the substrate of lumbar stenosis is the hypermotion, the only way to stop the degeneration is to block it; this goal is achieved through the bone fusion. So IFD’s aim is not motion preservation but bone fusion and the immobilization of the metamere. These devices have a double function, related to their possible association with TLIF interbody fusion[38-42].
Spinous process fusion of a spinal motor unit occurs after placement of the device in distraction or in neutral position. If the device is implanted in distraction, the biomechanical alteration persists because the axial load is altered, but the pathological segment is stabilized by the osseous fusion. The degenerative process can progress towards the adjacent segments with the development of an adjacent segment disease.
In my opinion, this is the best use for IFD. This surgery is recommended in cases of monolateral radiculopathy with foraminal stenosis due to facet hypertrophy. The surgical procedure includes artrectomy to perform a TLIF and complete decompression of the foramen and the nerve root, associated with the implant of a device in neutral position (not in distraction)[38-42].
This technique offers several advantages: (1) The execution of a TLIF allows performing a monolateral decompression and the insertion of an anterior intersomatic cage. The cage, in relationship with its width, can restore the physiological lumbar lordosis and leave the sagittal balance of the lumbar spine unaltered; (2) The insertion of the cage in the TLIF technique allows a higher fusion rate than the one obtained in a PLIF technique, in relationship with the most anterior position of the cage and of the width of the cage itself; (3) The insertion of the device in neutral position stabilizes the segment in its physiological position without distracting the segment; and (4) This procedure allows performing a circumferential fusion with an exclusively posterior and monolateral approach, preserving muscular insertions and the posterior tension band.
Recently, these devices have gone through an evolution, with the creation of expansion devices and cardanic compression devices that allow the distraction and the compression of the segment during the surgical procedure. These new devices allow modelling the orientation of the segment towards compression, increasing the pressure on the cage and assuring a better interbody fusion.
The surgical conditions in which restricted and non restricted IPD are recommended are fully described in the literature: foraminal and/or central stenosis, soft stenosis, I grade spondylolisthesis (actually debated), low back pain, black disc[64-69].
In the last few years, many authors have reported the high rate of surgical revision and symptom recrudescence in patients who have had these devices implanted[28-35,43-46,50]. In my opinion, from the literature review and my personal experience, surgical indications for the use of these interspinous devices are basically absent: (1) In foraminal stenosis, their only action is to accelerate the segmental degenerative process; (2) In central stenosis, they have no indication because their action is not resolutive for claudication, with its gold standard treatment the central decompression obtained with laminectomy; (3) In spondylolisthesis they are not indicated because the shear stress acting on the disc is high and the slippage would be augmented[28-35,43-46]; and (4) In low back pain due to micro-instability and in black disc conditions these devices would not be implanted because they do not reduce micro-instability but increase it, overloading the disc and augmenting pain.
In my opinion, interspinous devices have no clinical indication at the moment.
Interspinous stabilizers generating fusion, such as IFD, have a small range of surgical indications instead: monolateral or bilateral foraminal stenosis without evidence of spondylolisthesis in X-ray dynamic projections. These implants, which in my opinion have to be associated with TLIF and be inserted in neutral position or in slight compression, can allow the decompression of the stenotic nerve root with the TLIF technique, explicit in a slight compression supporting a contact between cage and endplates to promote a better intersomatic osseous fusion and promoting an interspinous and intersomatic osseous fusion, blocking the segmental degenerative process, responsible for the pathology and the symptoms[64-77].
The costs of the device and its surgical revision should not be underestimated. In a 2012 review concerning the post-op status of IPD, Epstein et al[74] reported a 11.6%-38% complication rate, 4.6%-85% reoperation rate and a 66.7%-77% incidence of poor outcomes. Furthermore, the cost of every single device is very high. So, high cost, high rate of complications, reoperation rates and poor outcomes make the choice of implantation of an IPD really controversial. In light of the points expressed previously, I think that IPD can be summarized as follows: highly expensive and poorly effective[64-69].
Dynamic neutralization systems should be studied, built and then implanted in order to preserve spinal biomechanics. The preservation of the physiological characteristics of the spine should particularly be aimed towards the whole motor unit (disc, facets, posterior tension band, ligaments) intended to be responsible for the segmental movement. IPD, as they are conceived today, do not seem to respect the biomechanical characteristics of the motor unit, accelerating the degenerative process and worsening the pathological process at the root of the clinical symptoms of patients. So this kind of device does not seem to have a definite and correct clinical indication at the moment. The IFD with their main aim as the treatment of the root of the pathological condition (instability) have a restricted range of clinical indications and their use can definitely be a source both for the patient and the surgeon. It is important to underline that these implants must not become a trend but only a weapon in the surgeon’s hands and, as with every weapon, is extremely dangerous in wrong hands. So the spinal surgeon is the only one who can decide when to use it and must know in detail the effects of this weapon to use it correctly with no damage for the patient.
The author wants to thank Professor R Delfini, MD, PhD, Chief of Neurosurgery A, University of Rome “Sapienza” and Dr. N Marotta, MD, PhD, neurosurgeon from Neurosurgery A, University of Rome “Sapienza”.
P- Reviewer: Lakhdar F, Kasai Y S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Przybyla AS, Skrzypiec D, Pollintine P, Dolan P, Adams MA. Strength of the cervical spine in compression and bending. Spine (Phila Pa 1976). 2007;32:1612-1620. [PubMed] [Cited in This Article: ] |
| 2. | Shirazi-Adl A. Analysis of large compression loads on lumbar spine in flexion and in torsion using a novel wrapping element. J Biomech. 2006;39:267-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Gillespie KA, Dickey JP. Biomechanical role of lumbar spine ligaments in flexion and extension: determination using a parallel linkage robot and a porcine model. Spine (Phila Pa 1976). 2004;29:1208-1216. [PubMed] [Cited in This Article: ] |
| 4. | Adams MA, Hutton WC, Stott JR. The resistance to flexion of the lumbar intervertebral joint. Spine (Phila Pa 1976). 1980;5:245-253. [PubMed] [Cited in This Article: ] |
| 5. | Gudavalli MR, Triano JJ. An analytical model of lumbar motion segment in flexion. J Manipulative Physiol Ther. 1999;22:201-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine (Phila Pa 1976). 1998;23:2552-2562. [PubMed] [Cited in This Article: ] |
| 7. | Hindle RJ, Pearcy MJ, Cross A. Mechanical function of the human lumbar interspinous and supraspinous ligaments. J Biomed Eng. 1990;12:340-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Anderson AL, McIff TE, Asher MA, Burton DC, Glattes RC. The effect of posterior thoracic spine anatomical structures on motion segment flexion stiffness. Spine (Phila Pa 1976). 2009;34:441-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Sharma M, Langrana NA, Rodriguez J. Role of ligaments and facets in lumbar spinal stability. Spine (Phila Pa 1976). 1995;20:887-900. [PubMed] [Cited in This Article: ] |
| 10. | Ploumis A, Christodoulou P, Kapoutsis D, Gelalis I, Vraggalas V, Beris A. Surgical treatment of lumbar spinal stenosis with microdecompression and interspinous distraction device insertion. A case series. J Orthop Surg Res. 2012;7:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Buric J, Pulidori M, Sinan T, Mehraj S. DIAM device for low back pain in degenerative disc disease : 24 months follow-up. Acta Neurochir Suppl. 2011;108:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Nardi P, Cabezas D, Rea G, Pettorini BL. Aperius PercLID stand alone interspinous system for the treatment of degenerative lumbar stenosis: experience on 152 cases. J Spinal Disord Tech. 2010;23:203-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Postacchini R, Ferrari E, Cinotti G, Menchetti PP, Postacchini F. Aperius interspinous implant versus open surgical decompression in lumbar spinal stenosis. Spine J. 2011;11:933-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Lauryssen C. Appropriate selection of patients with lumbar spinal stenosis for interspinous process decompression with the X STOP device. Neurosurg Focus. 2007;22:E5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Techy F, Mageswaran P, Colbrunn RW, Bonner TF, McLain RF. Properties of an interspinous fixation device (ISD) in lumbar fusion constructs: a biomechanical study. Spine J. 2013;13:572-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Gonzalez-Blohm SA, Doulgeris JJ, Aghayev K, Lee WE, Volkov A, Vrionis FD. Biomechanical analysis of an interspinous fusion device as a stand-alone and as supplemental fixation to posterior expandable interbody cages in the lumbar spine. J Neurosurg Spine. 2014;20:209-219. [PubMed] [Cited in This Article: ] |
| 18. | Karahalios DG, Kaibara T, Porter RW, Kakarla UK, Reyes PM, Baaj AA, Yaqoobi AS, Crawford NR. Biomechanics of a lumbar interspinous anchor with anterior lumbar interbody fusion. J Neurosurg Spine. 2010;12:372-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Tian NF, Zhang XL, Wu YS, Jiang LB, Xu HZ, Chi YL. Fusion after interspinous device placement. Orthopedics. 2012;35:e1822-e1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Pradhan BB, Turner AW, Zatushevsky MA, Cornwall GB, Rajaee SS, Bae HW. Biomechanical analysis in a human cadaveric model of spinous process fixation with an interlaminar allograft spacer for lumbar spinal stenosis: Laboratory investigation. J Neurosurg Spine. 2012;16:585-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Kaibara T, Karahalios DG, Porter RW, Kakarla UK, Reyes PM, Choi SK, Yaqoobi AS, Crawford NR. Biomechanics of a lumbar interspinous anchor with transforaminal lumbar interbody fixation. World Neurosurg. 2010;73:572-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14:491-504. [PubMed] [Cited in This Article: ] |
| 23. | Sairyo K, Biyani A, Goel V, Leaman D, Booth R, Thomas J, Gehling D, Vishnubhotla L, Long R, Ebraheim N. Pathomechanism of ligamentum flavum hypertrophy: a multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine (Phila Pa 1976). 2005;30:2649-2656. [PubMed] [Cited in This Article: ] |
| 24. | Kosaka H, Sairyo K, Biyani A, Leaman D, Yeasting R, Higashino K, Sakai T, Katoh S, Sano T, Goel VK. Pathomechanism of loss of elasticity and hypertrophy of lumbar ligamentum flavum in elderly patients with lumbar spinal canal stenosis. Spine (Phila Pa 1976). 2007;32:2805-2811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Zhong ZM, Zha DS, Xiao WD, Wu SH, Wu Q, Zhang Y, Liu FQ, Chen JT. Hypertrophy of ligamentum flavum in lumbar spine stenosis associated with the increased expression of connective tissue growth factor. J Orthop Res. 2011;29:1592-1597. [PubMed] [Cited in This Article: ] |
| 26. | Honsawek S, Poonpukdee J, Chalermpanpipat C, Payungporn S, Limthongkul W, Yingsakmongkol W, Thanakit V, Parkpian V. Hypertrophy of the ligamentum flavum in lumbar spinal canal stenosis is associated with increased bFGF expression. Int Orthop. 2013;37:1387-1392. [PubMed] [Cited in This Article: ] |
| 27. | Landi A, Delfini R. Soft stenosis of the lumbar spine : Thickness vs Hypertrophy of the ligamentum flavum. A patogenetic and molecular point of view. J Spine. 2013;2:e11. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Barbagallo GM, Olindo G, Corbino L, Albanese V. Analysis of complications in patients treated with the X-Stop Interspinous Process Decompression System: proposal for a novel anatomic scoring system for patient selection and review of the literature. Neurosurgery. 2009;65:111-119; discussion 119-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Epstein NE. X-Stop: foot drop. Spine J. 2009;9:e6-e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Bowers C, Amini A, Dailey AT, Schmidt MH. Dynamic interspinous process stabilization: review of complications associated with the X-Stop device. Neurosurg Focus. 2010;28:E8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Chung KJ, Hwang YS, Koh SH. Stress fracture of bilateral posterior facet after insertion of interspinous implant. Spine (Phila Pa 1976). 2009;34:E380-E383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Sobottke R, Schlüter-Brust K, Kaulhausen T, Röllinghoff M, Joswig B, Stützer H, Eysel P, Simons P, Kuchta J. Interspinous implants (X Stop, Wallis, Diam) for the treatment of LSS: is there a correlation between radiological parameters and clinical outcome? Eur Spine J. 2009;18:1494-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Hartmann F, Dietz SO, Kuhn S, Hely H, Rommens PM, Gercek E. Biomechanical comparison of an interspinous device and a rigid stabilization on lumbar adjacent segment range of motion. Acta Chir Orthop Traumatol Cech. 2011;78:404-409. [PubMed] [Cited in This Article: ] |
| 34. | Korovessis P, Papazisis Z, Lambiris E. The role of rigid vs. dynamic instrumentation for stabilization of the degenerative lumbosacral spine. Stud Health Technol Inform. 2002;91:457-461. [PubMed] [Cited in This Article: ] |
| 35. | Barbagallo GM, Corbino LA, Olindo G, Foti P, Albanese V, Signorelli F. The “sandwich phenomenon”: a rare complication in adjacent, double-level X-stop surgery: report of three cases and review of the literature. Spine (Phila Pa 1976). 2010;35:E96-100. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Chou D, Lau D, Hermsmeyer J, Norvell D. Efficacy of interspinous device versus surgical decompression in the treatment of lumbar spinal stenosis: a modified network analysis. Evid Based Spine Care J. 2011;2:45-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Sun HL, Li CD, Liu XY, Lin JR, Yi XD, Liu H, Lu HL. [Mid-term follow-up and analysis of the failure cases of interspinous implants for degenerative lumbar diseases]. Beijing Da Xue Xue Bao. 2011;43:690-695. [PubMed] [Cited in This Article: ] |
| 38. | Li CD, Sun HL, Yu ZR. [Biomechanical study of interspinous fixational effect on the stiffness of adjacent segments]. Beijing Da Xue Xue Bao. 2011;43:657-660. [PubMed] [Cited in This Article: ] |
| 39. | Le Huec JC, Aunoble S, Philippe L, Nicolas P. Pelvic parameters: origin and significance. Eur Spine J. 2011;20 Suppl 5:564-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 40. | Le Huec JC, Charosky S, Barrey C, Rigal J, Aunoble S. Sagittal imbalance cascade for simple degenerative spine and consequences: algorithm of decision for appropriate treatment. Eur Spine J. 2011;20 Suppl 5:699-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Le Huec JC, Saddiki R, Franke J, Rigal J, Aunoble S. Equilibrium of the human body and the gravity line: the basics. Eur Spine J. 2011;20 Suppl 5:558-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Faundez A, Roussouly P, Le Huec JC. [Sagittal balance of the spine: a therapeutic revolution]. Rev Med Suisse. 2011;7:2470-2474. [PubMed] [Cited in This Article: ] |
| 43. | Schulte TL, Hurschler C, Haversath M, Liljenqvist U, Bullmann V, Filler TJ, Osada N, Fallenberg EM, Hackenberg L. The effect of dynamic, semi-rigid implants on the range of motion of lumbar motion segments after decompression. Eur Spine J. 2008;17:1057-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Liu HY, Gu AQ, Zhu ZQ, Zhou J. [The efficacy and complication analysis of interspinous dynamic device (Wallis) in patients of degenerative lumbar disease]. Zhonghua Wai Ke Za Zhi. 2012;50:788-791. [PubMed] [Cited in This Article: ] |
| 45. | Kaulhausen T, Zarghooni K, Stein G, Knifka J, Eysel P, Koebke J, Sobottke R. The interspinous spacer: a clinicoanatomical investigation using plastination. Minim Invasive Surg. 2012;2012:538697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Maida G, Marcati E, Sarubbo S. Heterotopic ossification in vertebral interlaminar/interspinous instrumentation: report of a case. Case Rep Surg. 2012;2012:970642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Zucherman JF, Telles CJ. Commentary: Interspinous devices, spondylolisthesis, and spinous process-related complications. Spine J. 2012;12:473-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Krauss WE. Interspinous distraction devices: too good to be true? Yes. World Neurosurg. 2013;80:78-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Hsieh PC. Efficacy of surgical treatment for lumbar stenosis with the X-STOP: an issue in need of closer inspection. World Neurosurg. 2013;80:74-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Barz T, Lange J, Melloh M, Staub LP, Merk HR, Klöting I, Follak N. Histomorphometric and radiographical changes after lumbar implantation of the PEEK nonfusion interspinous device in the BB.4S rat model. Spine (Phila Pa 1976). 2013;38:E263-E269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Kim DH, Shanti N, Tantorski ME, Shaw JD, Li L, Martha JF, Thomas AJ, Parazin SJ, Rencus TC, Kwon B. Association between degenerative spondylolisthesis and spinous process fracture after interspinous process spacer surgery. Spine J. 2012;12:466-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Mayer HM. [Discogenic low back pain and degenerative lumbar spinal stenosis - how appropriate is surgical treatment?]. Schmerz. 2001;15:484-491. [PubMed] [Cited in This Article: ] |
| 53. | Siddiqui M, Karadimas E, Nicol M, Smith FW, Wardlaw D. Effects of X-STOP device on sagittal lumbar spine kinematics in spinal stenosis. J Spinal Disord Tech. 2006;19:328-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Korovessis P, Repantis T, Zacharatos S, Zafiropoulos A. Does Wallis implant reduce adjacent segment degeneration above lumbosacral instrumented fusion? Eur Spine J. 2009;18:830-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Kim KA, McDonald M, Pik JH, Khoueir P, Wang MY. Dynamic intraspinous spacer technology for posterior stabilization: case-control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg Focus. 2007;22:E7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Patil CG, Sarmiento JM, Ugiliweneza B, Mukherjee D, Nuño M, Liu JC, Walia S, Lad SP, Boakye M. Interspinous device versus laminectomy for lumbar spinal stenosis: a comparative effectiveness study. Spine J. 2014;14:1484-1492. [PubMed] [Cited in This Article: ] |
| 57. | Moojen WA, Arts MP, Jacobs WC, van Zwet EW, van den Akker-van Marle ME, Koes BW, Vleggeert-Lankamp CL, Peul WC. Interspinous process device versus standard conventional surgical decompression for lumbar spinal stenosis: randomized controlled trial. BMJ. 2013;347:f6415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Schizas C, Pralong E, Tzioupis C, Kulik G. Interspinous distraction in lumbar spinal stenosis: a neurophysiological perspective. Spine (Phila Pa 1976). 2013;38:2113-2117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Tian NF, Wu AM, Wu LJ, Wu XL, Wu YS, Zhang XL, Xu HZ, Chi YL. Incidence of heterotopic ossification after implantation of interspinous process devices. Neurosurg Focus. 2013;35:E3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Yao Q, Wang S, Shin JH, Li G, Wood K. Motion characteristics of the lumbar spinous processes with degenerative disc disease and degenerative spondylolisthesis. Eur Spine J. 2013;22:2702-2709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Xu C, Ni WF, Tian NF, Hu XQ, Li F, Xu HZ. Complications in degenerative lumbar disease treated with a dynamic interspinous spacer (Coflex). Int Orthop. 2013;37:2199-2204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Zang L, DU P, Hai Y, Su QJ, Lu SB, Liu T. Device related complications of the Coflex interspinous process implant for the lumbar spine. Chin Med J (Engl). 2013;126:2517-2522. [PubMed] [Cited in This Article: ] |
| 63. | Nachanakian A, El Helou A, Alaywan M. The interspinous spacer: a new posterior dynamic stabilization concept for prevention of adjacent segment disease. Adv Orthop. 2013;2013:637362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Kong DS, Kim ES, Eoh W. One-year outcome evaluation after interspinous implantation for degenerative spinal stenosis with segmental instability. J Korean Med Sci. 2007;22:330-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Kuchta J, Sobottke R, Eysel P, Simons P. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur Spine J. 2009;18:823-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Galarza M, Fabrizi AP, Maina R, Gazzeri R, Martínez-Lage JF. Degenerative lumbar spinal stenosis with neurogenic intermittent claudication and treatment with the Aperius PercLID System: a preliminary report. Neurosurg Focus. 2010;28:E3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Floman Y, Millgram MA, Smorgick Y, Rand N, Ashkenazi E. Failure of the Wallis interspinous implant to lower the incidence of recurrent lumbar disc herniations in patients undergoing primary disc excision. J Spinal Disord Tech. 2007;20:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Kim DH, Tantorski M, Shaw J, Martha J, Li L, Shanti N, Rencu T, Parazin S, Kwon B. Occult spinous process fractures associated with interspinous process spacers. Spine (Phila Pa 1976). 2011;36:E1080-E1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Verhoof OJ, Bron JL, Wapstra FH, van Royen BJ. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17:188-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Borg A, Nurboja B, Timothy J, Choi D. Interspinous distractor devices for the management of lumbar spinal stenosis: a miracle cure for a common problem? Br J Neurosurg. 2012;26:445-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Patil S, Burton M, Storey C, Glenn C, Marino A, Nanda A. Evaluation of interspinous process distraction device (X-STOP) in a representative patient cohort. World Neurosurg. 2013;80:213-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Shabat S, Miller LE, Block JE, Gepstein R. Minimally invasive treatment of lumbar spinal stenosis with a novel interspinous spacer. Clin Interv Aging. 2011;6:227-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Heyrani N, Picinic Norheim E, Elaine Ku Y, Nick Shamie A. Interspinous process implantation for the treatment of neurogenic intermittent claudication. Anesth Pain Med. 2012;2:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Epstein NE. A review of interspinous fusion devices: High complication, reoperation rates, and costs with poor outcomes. Surg Neurol Int. 2012;3:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Malter AD, McNeney B, Loeser JD, Deyo RA. 5-year reoperation rates after different types of lumbar spine surgery. Spine (Phila Pa 1976). 1998;23:814-820. [PubMed] [Cited in This Article: ] |
| 76. | Yuzawa Y. The interspinous ligament should be removed for the decompression surgery with the case of lumbar spinal canal stenosis. Arch Orthop Trauma Surg. 2011;131:753-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Kaulhausen T, Siewe J, Eysel P, Knifka J, Notermans HP, Koebke J, Sobottke R. The role of the inter-/supraspinous ligament complex in stand-alone interspinous process devices: a biomechanical and anatomic study. J Neurol Surg A Cent Eur Neurosurg. 2012;73:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |