Soil Carbon Sequestration Potential of Terrestrial Ecosystems: Trends and Soil Priming Effects
DOI: http://dx.doi.org/10.12944/CWE.17.1.14
Copy the following to cite this article:
Dinakaran J, Abbas N. S, Bhardwaj S, Kaula B. C. Soil Carbon Sequestration Potential of Terrestrial Ecosystems: Trends and Soil Priming Effects. Curr World Environ 2022;17(1). DOI:http://dx.doi.org/10.12944/CWE.17.1.14
Copy the following to cite this URL:
Dinakaran J, Abbas N. S, Bhardwaj S, Kaula B. C. Soil Carbon Sequestration Potential of Terrestrial Ecosystems: Trends and Soil Priming Effects. Curr World Environ 2022;17(1). Available From:
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 12-11-2021 |
|---|---|
| Accepted: | 02-02-2022 |
| Reviewed by: | 
 Delphine Derrien
Delphine Derrien
|
| Second Review by: |

 Marlín Pérez Suarez
Marlín Pérez Suarez
|
| Final Approval by: | Dr. Igor M. Danilin |
Introduction
The concentration levels of carbon dioxide (CO2) in the atmosphere plays a precarious role in maintaining the global surface temperature.1,2. The level of atmospheric CO2 concentration is increased at alarming rate after the industrial revolution across the world. As compared to the pre-industrial level (280 ppm), the current CO2 concentration is about ~414 ppm.3 A lot of studies have reported that the increase in concentrations of atmospheric CO2 is due to anthropogenic activities especially fossil fuel burning, deforestation, and agricultural management practices.4-7 The global surface temperature has increased by around 1.1°C in 2017 since 1850, i.e. above the preindustrial level, and it’s expected to increase at 0.2°C per decade.8 The escalation in the earth’s surface temperature affects the terrestrial ecosystem processes, ecosystem services and disturbs the terrestrial ecosystem carbon ?uxes.9 It’s essential to ascertain suitable scientific techniques or natural processes for alleviating atmospheric concentrations of CO2 in the terrestrial ecosystem. The United Nations climate entity is United Nations Framework Convention on Climate Change ( UNFCC) and several countries (both developed and developing) are its members and they have agreed to tackle the problem of global climate change by reducing the CO2 emissions from industrial activities. The Kyoto Protocol, consented by industrialized and unindustrialized countries in the year 1997, specified that the well-developed industrialized nations required to cut their CO2 emissions from industrial and other activities with reference to the 1990 level.10 Kyoto protocol, article 3.3 and 3.4, mentions that the industrialised nations (mostly developed) are required to cut some significant amount of carbon emissions by reforestation and afforestation programs and invest clean energy projects in the developing countries to curb CO2 emissions through clean development mechanism activities.10,11The Copenhagen and Durban climate accords are not successful especially to decide on a officially binding agreement to reduce the major greenhouse gases emissions by member countries. However, they agreed to focus on reduction of emissions from deforestation and forest degradation (REDD+).12 Major features of REDD+ are to reduce CO2 emissions from forests that suffer loss of vegetation cover and forest degradation activities and to increase sequestration potential of forests across the globe resulting in effective carbon sink.12 According to Paris climate accords (2015), all the member states of UNFCC agreed to make policies needed for sustainable future. Also, the agreement made a framework for controlling global warming well below 1.5°C by the end of this century.8 Carbon sequestration in terrestrial ecosystems, by plantation activities, is considered as the best approach to absorb significant amount of atmospheric carbon and also to reduce land degradation. There is a scarcity of data on the impacts of afforestation and plantation activities on carbon sink and sequestration potential across the world.13,14 Besides an earlier study15 showed the importance of agricultural management practices especially no tillage, organic manure amendments and nitrogen fertilization on organic carbon storage in soils. There have been several studies reported that the fresh carbon substrates (via photosynthates) accelerate the already stored carbon i.e. called priming effect. An attempt has been made in this review to presents soil carbon sequestration potential of terrestrial ecosystem and the significance of soil priming effect.
Global and Indian Forest Cover
On a global scale, forests comprise of about 4.06 billion hectares i.e. 30.8% of the complete land area.16 More than 50% of the global forests are situated in United States of America (USA), Canada, the Russian Federation, Brazil etc.17 The total forest cover of India is 24.56% of the geographical area which also includes the tree outside forest cover (2.89%) of the country.18 According to an earlier study, out of 328.7 million hectares of the total geographical area of India, 161.8, 57, 68.35, 11.05, and 7.95 million hectares are of arable land, irrigated land, forest land, permanent pasture, and permanent croplands respectively.19 Hence, increasing agricultural activities through deforestation in temperate, tropical, arid and semiarid regions have a more profound effect on terrestrial ecosystem carbon flux. Hence an earlier study7 estimated the net carbon flux due to land use and land cover change for the period 1850-2015 was 145±16 Pg C and more from the tropical regions (102±5.8 Pg C) of the world. Indian forests (the Western Ghats and Himalayan forests) are considered as one of the major biodiversity hotspots of the world. Therefore, Indian forest ecosystems plays a crucial role in regulating carbon cycle at regional and global level.
Carbon Storage in the Terrestrial Forest Ecosystems
In the earth’s terrestrial ecosystems, forest ecosystems occupy a significant part and play a central role in the terrestrial carbon cycle.14 Forest ecosystems, around the world, stored higher amounts of organic carbon in above and below-ground parts with longer residence times especially in the soil via the carbon sequestration process.20 Globally, forest ecosystems absorb a lot of atmospheric CO2 through the process of photosynthesis. The forest ecosystem releases an almost equal amount of CO2 into the atmosphere via the respiration process.20 However, a significant amount of carbon is stored in forests vegetation biomass, dead twigs, leaves, detritus matters, and soil.20 Therefore, forest ecosystems are considered an important carbon sink of terrestrial ecosystems. World forest ecosystems occupy about 4.06 billion hectares and act as a reservoir of carbon.17,21 Research studies22,23 reported that tropical, temperate and boreal forests sequestered about 55-63%, 26-31% and 11-14% of the total carbon stock of the world’s forests respectively (Figure 1). Tropical forests occupy about 1.76 million hectares and are considered as nature’s green engines of our planet earth.24 The soils of tropical forests stored three folds more carbon as compared to the aboveground vegetation biomass. Thus tropical forests store 471±93 PgC (Pg = petagram) and they can store up to 120-194 Mg ha-1 of carbon.14,24 Tropical forests are very dynamic in terms of plant growth, mineralization, and litter decomposition because of their unique climatic conditions.25,26 It has been reported that deforestation and forest degradation in tropics to release about 0.5 to 3.5 Pg C yr-1.27-29. Also it has been reported that tropical trees accomplish 60% of the world photosynthesis and release almost similar amount via litter decomposition by microbes.27 An earlier study30 reported that the age of soil carbon, in tropical forests and grasslands, increases (7 to 1250 years) with an depth increases. For example, 45% of topsoil carbon (0-30cm) comes under the age of 50 years. Therefore, small changes in the carbon stocks in the tropical forests may alter the earth's carbon cycling. Furthermore, tropical forests are more fragile, due to the climatic conditions, and they respond so rapidly, if they are under environmental stress conditions, to the global carbon cycle.28 Global forests and soils together stored about 1240 PgC.21 Soils are considered as a potential reservoir of carbon in the terrestrial ecosystem of our planet earth. Global soils are considered as a major sink or source of CO2 with the subject to conventional and/or different types of agricultural management activities.14
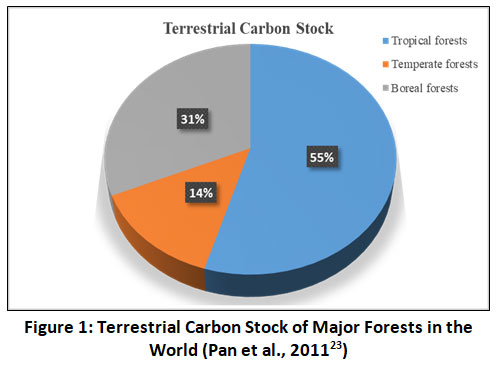 |
Figure 1: Terrestrial Carbon Stock of Major Forests in the World (Pan et al., 201123) Click here to view Figure |
In the year 2009, the total carbon stocks of Indian forests, trees and soils was estimated to be about 6621 million tonnes.18 The recent FSI report (2019) states that Indian forests stored about 7124.6 million tonnes carbon. Among different forest types in India, tropical dry deciduous forests stored the maximum amount of carbon (2158 million tonnes carbon) followed by other types of forests.18 Tropical dry deciduous forests occupies about 40% of total forest cover in India.18 Moreover tropical dry deciduous forest soils can act as a major sink for carbon for longer time periods 31,32 (Figure 2).
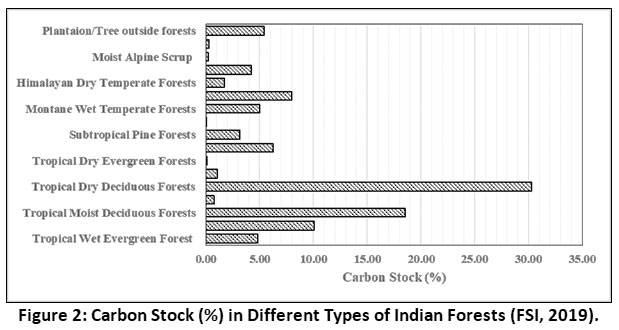 |
Figure 2: Carbon Stock (%) in Different Types of Indian Forests (FSI, 2019). Click here to view Figure |
According to FAO29, decreasing forest areas due to anthropogenic activities in the recent decade come down because of policies and actions taken by many of the developed and developing countries. Especially, afforestation or plantation activities may play a greater role in ecosystem restoration and increasing forest cover. According to the 4 per mille concept, every nation should increase their total soil carbon storage capacity by 0.4% per year to compensate the global emission of greenhouse gases.33 The forest and tree cover of the country had increased about 0.65% (5,188 sq km) during the FSI assessment period from 2017 to 2019.18,34 Besides, Indian forests stored about 7124.6 million tons of carbon in five different components viz., biomass lying above and below the ground, debris, vegetation litter and soil.18 Among them, soil stored 56.19% (4003.6 million tons) of carbon.18 Also they found an increase in carbon stock (21.3 million tons) from 2017 to 2019. Thus accurate measurement of carbon stock in five different components, temporality, in different forest ecosystem is also very important.
Soil Organic Carbon (SOC) Pools
The CENTURY-C and ROTH-C carbon models are effectively applied to understand the changes of SOC pools in agricultural and forest soils.35,36 The ROTH-C and CENTURY-C model has different organic carbon pools based on their turnover time. For example, the CENTURY-C model has three different pools viz., active, slow and passive.35 Whereas the ROTH-C model has five different pools viz., Decomposable Plant Material (DPM), Resistant Plant Material (RPM), Microbial Biomass (BIO) and Humified Organic Matter (HUM) and Inert Organic Matter (IOM).37 Above mentioned pools are not directly quantifiable, instead they are conceptual pools. Thus several researchers have tried to fractionate different soil organic matter pools based on particle size (sand, silt and clay fractions) and chemical (labile or mineral associated/recalcitrant).38 Understanding the changes of soil organic carbon pools in terrestrial ecosystem becomes imperative to develop new sustainable or management practices to store more carbon in the soils. Table 1 shows the SOC content in different particles and chemical fractions. A combination of physical and chemical fractionation method is most effective in separation of organic carbon fractions with different turnover rates from soils.39 However, still there is no discrete method or technique available to separate the organic carbon fractions with different turnover rates in all types of soils.
Table 1: Fractions of Soil Organic Carbon Content in Different Land Use and Cover Types.
|
No |
Name of Fractions |
SOC |
Reference |
|
1. |
Particle size fractions Tropical forest in Costa Rica, Gallery Secondary forest
15-year-old Secondary forest
25-year-old Secondary forest
Abandoned plantation (>60 years old)
|
SOC (%) <20µm :34.7% 20-53 µm :23.2% 53-105 µm :23.8% 105-200 µm :18.3% <20µm :29.2% 20-53 µm :26.0% 53-105 µm :24.4% 105-200 µm :20.4% <20µm :30.5% 20-53 µm :25.7% 53-105 µm :23.8% 105-200 µm :19.9% <20µm :34% 20-53 µm :27.1% 53-105 µm :21.6% 105-200 µm :17.2% |
40
|
|
2. |
Different vegetation covers in north eastern china
Heavy fractions
Resistant fractions |
0-20cm TSOC 2.1-66.6 g kg-1 20-40cm 0.8-46.1 g kg-1
0-20cm 1.8-65 g kg-1 20-40cm 0.7-45.8 g kg-1
0-20cm 0.7-54.4g kg-1 20-40cm 0.3-37.3 g kg-1 |
41
|
|
3. |
Chemical fractionation Fulvic acid
Humic acid
Humin
Total SOC |
Mardi watershed, Nepal Forest :01.36 kg Cm-2 Grass land :01.59 kg Cm-2 Agricultural land :0.80 kg Cm-2
Forest :01.82 kg Cm-2 Grass land :01.45 kg Cm-2 Agricultural land :0.57 kg Cm-2
Forest :02.25 kg Cm-2 Grass land :01.72 kg Cm-2 Agricultural land :01.44 kg Cm-2
Forest :06.13 kg Cm-2 Grass land :05.03 kg Cm-2 Agricultural land :02.84 kg Cm-2 |
42
|
|
4. |
Physical fractionation Particulate organic matter >53 - 250 µm (POM/TOC) |
0-15 cm Sandy loam Monocropping :39.8% Agroforestry :26.5% Sandy clay loam Mono-cropping: 17.9% Agroforestry : 25.2% |
43
|
|
5. |
Physical fractionation POM
Mineral associated carbon (<53 µm) |
Agramunt site ( 0-40 cm) No tillage :17.9Mg ha-1 Reduced tillage :13.8Mg ha-1 Sub-soil tillage : 14.0Mg ha-1 Conventional tillage : 15.4Mg ha-1 Selvanera site No tillage :11.5Mg ha-1 Reduced tillage :12.5Mg ha-1 Sub-soil tillage : 14.9Mg ha-1 Conventional tillage : 17.0Mg ha-1 Penaflor in continuous barley cropping system No tillage :08.2Mg ha-1 Reduced tillage :06.0Mg ha-1 Conventional tillage :05.7Mg ha-1
Agramunt site No tillage :28.8Mg ha-1 Reduced tillage :32.4Mg ha-1 Sub-soil tillage :30.0Mg ha-1 Conventional tillage :31.2Mg ha-1
Selvanera site No tillage :44.5Mg ha-1 Reduced tillage :48.6Mg ha-1 Sub-soil tillage :48.4Mg ha-1 Conventional tillage :46.2Mg ha-1 Penaflor in continuous barley cropping system No tillage :42.2Mg ha-1 Reduced tillage :42.1Mg ha-1 Conventional tillage :41.8Mg ha-1 |
44
|
|
6
|
Density fractionation
Light fraction
Heavy fraction
|
0-20 cm Conventional tillage :42.2 g kg-1 No tillage :44.1g kg-1 Forest (oak forest) :86.9 g kg-1
Conventional tillage :7.9 g kg-1 No tillage :9.8 g kg-1 Forest (oak forest) :20.4 g kg-1 |
45 |
|
7 |
Particle size fractionation Coarse-silt (63-20µm)
Medium-silt (20-6.3 µm)
Fine-silt (6.3-2 µm)
|
Arable land top-soil :1750 mgC kg-1 Arable land sub-soil :530 mgC kg-1 Grassland top-soil :1700 mgC kg-1 Grassland sub-soil :430 mgC kg-1 Woodland top-soil :8900 mgC kg-1 Woodland sub-soil :8470 mgC kg-1 Arable land top-soil :6400 mgC kg-1 Arable land sub-soil :1060 mgC kg-1 Grassland top-soil :9770 mgC kg-1 Grassland sub-soil :730 mgC kg-1 Woodland top-soil :28870 mgC kg-1 Woodland sub-soil :19270 mgC kg-1 Arable land top-soil :4850 mgC kg-1 Arable land sub-soil : 1800 mgC kg-1 Grassland top-soil :5290 mgC kg-1 Grassland sub-soil :1610 mgC kg-1 Woodland top-soil :14970 mgC kg-1 Woodland sub-soil :10440 mgC kg-1 |
46
|
Priming Effect (PE)
The change in the rate of decomposition of already stored organic carbon (loss of carbon) when you supply the fresh carbon substrates into the soils is termed as priming effect. 47-49 It is categorized as real priming effect (loss of carbon) and apparent priming effect (extra CO2 production by microbial populations).50 Globally, several studies have reported that freshly added carbon substrate accelerates the priming effect (loss of native carbon) through rejuvenation of microbial populations and their biomass turnover.47-50 Besides, the freshly added carbon substrates (rhizodeposits or fine roots) control the alterations in the growth of microbial populations and in turn affect the real priming effect or no effect on already stored carbon in the soils.51-55 These alterations in the decomposition of stored carbon depend on the nutrient composition and /or budget of the particular soil.51,56 An earlier study48 hypothesized that r-strategists types of microbes dominate till the easily utilizable substrates (like glucose) are exhausted in the soil system. After that, the gradual change from r-strategists to the k-strategists group of microbes dominate to decompose the resistant carbon in the soils. Thus there is a competition between the fast-growing microbes i.e. r-strategists (utilize the easily decomposable substrate like glucose etc.) and k-strategists for utilization of the substrates in the soil.56
The easily utilizable substrates are exhausted in the soil then the r-strategists become dormant and the k-strategists dominate in the system to decompose the insoluble organic compounds available in the soil organic matter.50 The real priming effect would not occur even after the addition of easily utilizable substrates into the soil, because r-strategists may dominate in the system but they are unable to utilize the native soil organic matter. Thus an earlier study50 suggested that such priming effect may be “apparent” which is due to accelerated microbial biomass rather than by decomposition of already stored and /or build in soil organic matter. The acceleration of organic matter decomposition is totally dependent upon the living (microbes) and dead organic components. The microbial biomass acts as one of the important soil organic matter pools besides carbon and nitrogen in the terrestrial soil ecosystem.57 A small change in the quantity and/or turnover rate of various soil organic pools, especially labile and recalcitrant, may have a greater impact on the total carbon budget of regional to global scale level.
Conclusion
Understanding the carbon sequestration potential of natural forests, abandoned lands, grasslands, agricultural lands, and plantations is very crucial to store the atmospheric carbon in the terrestrial ecosystem. The selection of native plant species for the plantation activities, in any region, is essential in order to maintain the sustainable ecosystem process. It is a challenge to assess the soil-carbon sink and carbon sequestration potential strategy of afforestation activities of any region in the world. A multidisciplinary approach (climatologists, ecologists, geologists, etc.) is required to understand the mechanisms of soil-carbon sink relationship and sequestration potential of any terrestrial ecosystem. An accurate estimation of carbon stock and monitoring the factors that affect the storage of carbon in forests and plantation soils are more important while assessing the total carbon stock of any ecosystem. It is essential to understand the changes of already stored organic matter pools in different types of forest ecosystems and plantations. Thus, more research analysis is warranted to comprehend the positive or negative priming effect of already stored carbon in terrestrial ecosystems.
Acknowledgements
The authors are thankful to Prof. Balaram Pani, Principal of Bhaskaracharya College of Applied Sciences, University of Delhi, and Prof. K.K. Arora, Principal, Zakir Husain Delhi College, University of Delhi, Delhi for the encouragement, support and motivation.
Funding Source
Authors haven’t received any financial assistance for writing and publishing the paper.
Conflict of Interest
The authors do not have any conflict of interest.
References
- Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA; 2001: pp.881.
- Masson-Delmotte V, Zhai P, Pörtner HO, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Péan C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T. Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty.2018. https://www.ipcc.ch/sr15/.
- National Oceanic and Atmospheric Administration (NOAA). Monthly Average Mauna Loa CO2. 2021. https://gml.noaa.gov/ccgg/trends/.
- Lal R. Offsetting China's CO2Emissions by Soil Carbon Sequestration. Clim Change. 2004; 65(3):263-275.
CrossRef - Malhi Y, Roberts J, Betts R, Killeen T, Li W, Nobre C. Climate Change, Deforestation, and the Fate of the Amazon. Science. 2008; 319(5860):169-172.
CrossRef - National Academy of Sciences. Climate Change: Evidence and Causes: Update 2020. https://royalsociety.org/topics-policy/projects/climate-change-evidence-causes/
- Houghton R, Nassikas A. Global and regional fluxes of carbon from land use and land cover change 1850-2015. Global Biogeochem Cycles. 2017; 31(3):456-472. doi:10.1002/2016gb005546.
CrossRef - Allen MR., Dube OP, Solecki W, Aragon-Durand F, Cramer W, Humphreys S, Kainuma M, Kala J, Mahowald N, Mulugetta Y, Perez R, Wairiu M, Zickfeld K. Framing and Context. In: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty [Masson-Delmotte V, Zhai O, Portner HO, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Pean C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield Y. (eds.)]. 2018. https://www.ipcc.ch/sr15/.
- Luo Y. Terrestrial Carbon–Cycle Feedback to Climate Warming. Annu Rev Ecol Evol Syst. 2007; 38(1):683-712.
CrossRef - Lau L, Lee K, Mohamed A. Global warming mitigation and renewable energy policy development from the Kyoto Protocol to the Copenhagen Accord—A comment. Renew. Sustain Energy Rev. 2012; 16(7):5280-5284.
CrossRef - Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA. IPCC 2007: Climate change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. pp.863.
- Graham K, Vignola R. REDD+ and agriculture: a cross-sectoral approach to REDD+ and implications for the poor. REDD-Net publications at Overseas Development Institute (ODI). London, UK. T: www. redd-net.org.
- Lal R. Soil carbon sequestration to mitigate climate change. Geoderma. 2004; 123(1-2):1-22.
CrossRef - Lal R. Forest soils and carbon sequestration. For Ecol Manage. 2005; 220(1-3):242-258.
CrossRef - Chenu C, Angers D, Barré P, Derrien D, Arrouays D, Balesdent J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil and Tillage Research. 2019;188:41-52. doi:10.1016/j.still.2018.04.011.
CrossRef - FAO. Global Forest Resources Assessment 2020 – Key findings. 2020. Rome. https://doi.org/10.4060/ca8753en.
CrossRef - FAO and UNEP. The State of the World’s Forests 2020. Forests, biodiversity and people. 2020. Rome. https://doi.org/10.4060/ca8642en.
CrossRef - Forest Survey of India (FSI). India state of the forest report 2019. Ministry of Environment Forest and Climate Change, Dehradun. Allied Printers, Dehradun. 2019.
- Lal R. Soil Carbon Sequestration in India. Clim Change. 2004; 65(3):277-296.
CrossRef - Lorenz K, Lal R. Carbon sequestration in forest ecosystems. 2010. Springer Dordrecht Heidelberg London New York, pp.27.
CrossRef - Dixon R, Solomon A, Brown S, Houghton R, Trexier M, Wisniewski J. Carbon Pools and Flux of Global Forest Ecosystems. Science. 1994; 263(5144):185-190.
CrossRef - Prentice IC. The Carbon Cycle and Atmospheric Carbon Dioxide. Climate Change 2001: The Scientific Basis IPCC, Cambridge University Press, Cambridge, UK. 2001; 183–237.
- Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P. A large and persistent carbon sink in the world’s forests. Science. 2011; 333(6045):988-93.
CrossRef - Food and Agriculture Organization of the United Nations FAO. Global Forest Resources Assessment. Main Report. FAO, 2000; Rome, Italy. Pp.512.
- Aerts R, Chapin FS. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv Ecol Res. 2000; 30: 1-67.
CrossRef - De Deyn G, Cornelissen J, Bardgett R. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett. 2008; 11(5):516-531.
CrossRef - Mitchard E. The tropical forest carbon cycle and climate change. Nature. 2018; 559(7715):527-534. doi:10.1038/s41586-018-0300-2.
CrossRef - Malhi Y, Phillips O. Tropical Forests and Global Atmospheric Change. Oxford: Oxford University Press; 2007. pp. 260.
- Food and Agriculture Organization of the United Nations (FAO). State of the world’s forests. Published by the Office of Knowledge Exchange, Research and Extension, FAO, Viale delle Terme di Caracalla, 00153 Rome, Italy. 2011.
- Balesdent J, Basile-Doelsch I, Chadoeuf J, Cornu S, Derrien D, Fekiacova Z, Hatté C. Atmosphere–soil carbon transfer as a function of soil depth. Nature. 2018; 559(7715):599-602.
CrossRef - Raha D, Dar J, Pandey P, Lone pa, Verma S, Khare PK, Khan ML. Variation in tree biomass and carbon stocks in three tropical dry deciduous forest types of Madhya Pradesh, India. Carbon Manag. 2020;11(2):109-120. doi:10.1080/17583004.2020.1712181.
CrossRef - Mehta N, Dinakaran J, Patel S, Laskar AH, Yadava MG, Ramesh R, Krishnayya NSR. Changes in litter decomposition and soil organic carbon in a reforested tropical deciduous cover (India). Ecol Res. 2012;28(2):239-248. doi:10.1007/s11284-012-1011-z.
CrossRef - Minasny B, Malone BP, McBratney AB, Angers DA, Arrouays D, Chambers A, Chaplot V, Chen ZS, Cheng K, Das BS, Field DJ. Soil carbon 4 per mille. Geoderma. 2017; 292:59-86.
CrossRef - Forest Survey of India (FSI). India state of the forest report 2017. Ministry of Environment Forest and Climate Change, Dehradun. Allied Printers, Dehradun. 2017.
- Parton W, Schimel D, Cole C, Ojima D. Analysis of Factors Controlling Soil Organic Matter Levels in Great Plains Grasslands. Soil Sci Soc Am J. 1987; 51(5):1173-1179.
CrossRef - Bhattacharyya T, Pal DK, Easter M, Williams S, Paustian K, Milne E, Chandran P, Ray SK, Mandal C, Coleman K, Falloon P. Evaluating the Century C model using long-term fertilizer trials in the Indo-Gangetic Plains, India. Agric Ecosyst Environ. 2007; 122(1):73-83.
CrossRef - Coleman K, Jenkinson DS. ROTHC-26.3 A model for the turnover of carbon in soil. Model description and windows users guide. 2005. Available at http://www.rothamsted.bbsrc.ac.uk/aen/carbon/ mod26_3_win.pdf.
- Lutzow MV, Knabner IK, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, Marschner B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem. 2007; 39: 2183-2207.
CrossRef - Poeplau C, Don A, Six J et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils – A comprehensive method comparison. Soil Biology and Biochemistry. 2018;125:10-26. doi:10.1016/j.soilbio.2018.06.025.
CrossRef - Jiménez JJ, Lal R, Russo RO, Leblanc HA. The soil organic carbon in particle-size separates under different regrowth forest stands of north eastern Costa Rica. Ecol Eng. 2008; 34(4):300-310.
CrossRef - Qing-Biao WU, Xiao-Ke WA, Ouyang ZY. Soil organic carbon and its fractions across vegetation types: Effects of soil mineral surface area and microaggregates. Pedosphere. 2009; 19(2):258-64.
CrossRef - Yang ZH, Singh BR, Sitaula BK. Soil organic carbon fractions under different land uses in Mardi watershed of Nepal. Commun Soil Sci Plant Anal. 2004; 35(5-6):615-29.
CrossRef - Mao R, Zeng DH, Li LJ, Hu YL. Changes in labile soil organic matter fractions following land use change from monocropping to poplar-based agroforestry systems in a semiarid region of Northeast China. Environ Monit Assess. 2012; 184(11):6845-53.
CrossRef - Álvaro-Fuentes J, López Sánchez MV, Cantero-Martínez C, Arrúe JL. Tillage effects on soil organic carbon fractions in Mediterranean dryland agroecosystems. Soil Sci Soc Am J. 2008; 72:541-547.
CrossRef - Tan Z, Lal R, Owens L, Izaurralde RC. Distribution of light and heavy fractions of soil organic carbon as related to land use and tillage practice. Soil Tillage Res. 2007; 92(1-2):53-9.
CrossRef - Adisa SJ, Nortcliff S. Carbon fractions associated with silt?size particles in surface and subsurface soil horizons. Soil Sci Soc Am J. 2011; 75(1):79-91.
CrossRef - Fontaine S, Mariotti A, Abbadie L. The priming effect of organic matter: a question of microbial competition?. Soil Biol Biochem. 2003; 35(6):837-43.
CrossRef - Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature. 2007; 450(7167):277-80.
CrossRef - Keuper F, Wild B, Kummu M, Beer C, Blume-Werry G, Fontaine S, Gavazov K, Gentsch N, Guggenberger G, Hugelius G, Jalava M. Carbon loss from northern circumpolar permafrost soils amplified by rhizosphere priming. Nat Geosci. 2020; 13(8):560-565.
CrossRef - Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol. 2007; 37(1-2):95-105.
CrossRef - Kuzyakov Y. Factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci. 2002; 165(4):382-96.
CrossRef - Zhu Z, Zeng G, Ge T, Hu Y, Tong C, Shibistova O, He X, Wang J, Guggenberger G, Wu J. Fate of rice shoot and root residues, rhizodeposits, and microbe-assimilated carbon in paddy soil–part 1: decomposition and priming effect. Biogeosciences. 2016; 13:4481-4489.
CrossRef - Weng ZH, Van Zwieten L, Singh BP, Tavakkoli E, Kimber S, Morris S, Macdonald LM, Cowie A. The accumulation of rhizodeposits in organo-mineral fractions promoted biochar-induced negative priming of native soil organic carbon in Ferralsol. Soil Biol Biochem. 2018; 118:91-96.
CrossRef - Zhou J, Zang H, Loeppmann S, Gube M, Kuzyakov Y, Pausch J. Arbuscular mycorrhiza enhances rhizodeposition and reduces the rhizosphere priming effect on the decomposition of soil organic matter. Soil Biol Biochem. 2020; 140:107641.
CrossRef - Kuzyakov Y, Friedel JK, Stahr K. Review of mechanisms and quantification of priming effects. Soil Biol Biochem. 2000; 32(11-12):1485-98.
CrossRef - Dorodnikov M, Blagodatskaya E, Blagodatsky S, Fangmeier A, Kuzyakov Y. Stimulation of r-vs. K-selected microorganisms by elevated atmospheric CO 2 depends on soil aggregate size. FEMS Microbiol Ecol 2009; 69(1):43-52.
CrossRef - Kuzyakov Y. Priming effects: interactions between living and dead organic matter. Soil Biol Biochem. 2010; 42(9):1363-71.
CrossRef







