Introduction
The first few months of seeding growth and seed germination are critical periods in the established of any plant species. Variations in temperature, water stress and light conditions significantly impact seed germination1. One of the leading environmental factors contributing to the degradation of agricultural land and a decline in crop productivity is soil salinity. Salinity is a crucial abiotic element that negatively impacts crops and yield2. High salinity reduces the osmotic potential of ambient soil water and tends to result in less water uptake by dry seeds. The process of seed germination ends when the radicle protrudes. Salt stress affects soil productivity and crop yield in dry and semi-arid regions3. Salinity stress has a significant negative effect on crop plants’ physiology and performance, and as a result, growth is arrested, and metabolic damage results in plant death4. Minimized dry matter accumulation by plants and grain production is the ultimate impact of salt stress on plants. When cultivated in salty soils, most plants accumulate high sodium (Na+) ions in their shoot tissues.
As a result, plants exhibit toxic consequences due to Na+ accumulation5.In India, soil salinity is the most significant and enduring danger to irrigated agriculture. In India, over 6.74 million hectares of land have been reported to be salt affected with states of Gujrat, U.P., Maharastra, West Bengal and Rajasthan accounting for almost 75 % of saline soils in the country. Agricultural production is greatly affected by soil salinity. One of the oldest oilseed crops, Sesame (Sesamum indicum L.), is cultivated extensively in Africa and Asia for its nutritious seeds. Sesame is often referred to as the “Queen of Oil Seeds” due to its exceptional properties. For thousands of years, people have utilized it to make edible oil, paste, cakes, and confections6.
Taking into account the details as mentioned earlier, the investigation was conducted to evaluate the effect of salinity on two sesame varieties by assessing germination efficiency and seedling health under simulated salt stress conditions at laboratory conditions.
Materials and Methods
This investigation was performed in March 2020 using sterilized glass Petri dishes with a tight-fitting lids to examine the germination behaviour of sesame cultivars under different salt stresses. Sterilized filter paper lined with thin cotton pads was kept on the Petri dishes. For seed germination, filter sheets were utilized as a matrix. Seeds of two sesame cultivars (GT-10 and JTS-8) were obtained from the Bihar Agricultural University at Sabour in Bhagalpur. Both cultivars’ uniformly sized seeds were picked, surface sterilized in a 2% sodium hypochlorite solution, and washed several times in distilled water. For two treatments, ten seeds were selected for each replicate. These filters were saturated with NaCl and Na 2SO4 solutions of known concentrations (Control, 0.05M, 0. I.M., 0.I5 M, 0.2M, 0.25M, 0.3M, 0.35M, 0.4M and 0.5M). We regarded the seeds to have germinated when the radicles began to emerge, and after soaking for 24 to 144 hours, these seeds were scored daily at definite intervals. The average germination rate was determined by averaging the results of several separate experiments. The radicle and hypocotyl lengths were measured individually in cm. 96 hours later. Radicles, hypocotyls, and cotyledons were measured on a digital balance for fresh weights. The Petri dishes were kept in the incubator at 28°C.
Results and Discussion
The NaCl solution significantly altered both cultivars’ germination rate and early growth. A study showed that the germination rate for GT-10 dropped from 95% to 40%, and JTS-8 dropped from 60% to 15%. Seeds of both cultivars failed to germinate above a concentration of 0.2M NaCl solution. With an increased NaCl concentration, seedling length and fresh weight steadily decreased (table-1-3 and fig.-1&2). The JTS-8 showed more salt tolerance than GT-10 having maximum seedling fresh weight (8.594 mg) and seedling length (0.655 cm.), whereas GT-10 had a maximum (6.301 mg) and seedling length (0.87cm.) after 144 hours under 0.2 M NaCl solution concentrations.
Salt stress causes reduced seedling growth7, 8. After 96 hours of germination, seeds of both cultivars showed similar results, demonstrating that seeds could germinate well at lower concentrations of NaCl (0.5 M)9. Numerous studies have demonstrated that higher NaCl concentrations can reduce plant germination and growth10, 11, 12, 13. It was discovered that the deleterious effects of soil salinity on crops occur at critical growth stages and, in extreme cases, result in a reduction of the crop’s overall yield.14
Because of the Na2SO4 solution’s stress, the germination percentage for GT-10 dropped from 95% to 15%, while for JTS-8, it dropped from 65% to 10%. (table-4-6 and fig.-3&4).In both cultivars, there is no germination above 0.3M concentration. For GT-10 and JTS-8, the seedling growth was shown up to 0.2 M concentration and 0.15 M concentration after 144 hours, respectively. Length and fresh weight of seedlings steadily decreased in both cultivars with an increase in solution concentration15. The JTS-8 exhibited a more severe effect that could not tolerate beyond 0.15 M salt concentration than GT-10. In this regard, it was observed that in most cases, the stress, whether it might be from NaCl or Na2SO4, adversely affected elongates of radicle16. They also discovered the deleterious impact of stress and took note of the variation in chloride or sulphate ion magnitude. Due to the harmful effects of NaCl and Na2SO4 and the seedling’s unbalanced nutritional intake, radicle length and weight decreased. Numerous researchers have found that crop seedlings grow more slowly under situations of salt stress, supporting the conclusion of this study17-21.
Table 1: Effect of Different NaCl Concentrations on the Germination percentage of Sesame Cultivars (GT-10 and JTS-8) Germination %
|
Treatment |
GT-10 |
JTS-8 |
| Control | 95 | 60 |
| 0.05M | 65 | 40 |
| 0.1M | 55 | 30 |
| 0.15M | 50 | 20 |
| 0.2M | 40 | 15 |
| 0.25M | — | — |
| 0.3M | — | — |
| 0.35M | — | — |
| 0.4M | — | — |
| 0.5M | — | — |
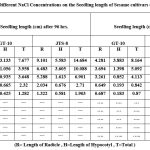 |
Table 2: Effect of Different NaCl Concentrations on the Seedling length of Sesame cultivars (GT-10 and JTS-8). |
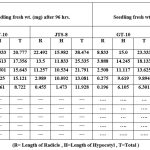 |
Table 3: Effect of Different NaCl Concentrations on the Seedling Fresh Weight of Sesame cultivars (GT-10 and JTS-8). |
Table 4: Effect of Different Na2SO4 concentrations on the Germination percentage of Sesame Cultivars (GT-10 and JTS-8) Germination %
| Treatment | GT-10 | JTS-8 |
| Control | 95 | 65 |
| 0.05M | 65 | 50 |
| 0.1M | 60 | 45 |
| 0.15M | 50 | 30 |
| 0.2M | 40 | 25 |
| 0.25M | 35 | 20 |
| 0.3M | 15 | 10 |
| 0.35M | — | — |
| 0.4M | — | — |
| 0.5M | — | — |
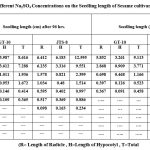 |
Table 5: Effect of Different Na2SO4 Concentrations on the Seedling length of Sesame cultivars (GT-10 and JTS-8). |
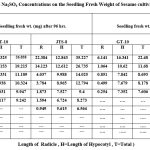 |
Table 6: Effect of Different Na2SO4 Concentrations on the Seedling Fresh Weight of Sesame cultivars (GT-10 and JTS-8). |
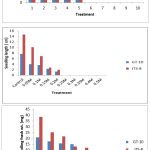 |
Figure 1: Effect of Different NaCl Concentrations on Germination Percentage (%), Seedling Length (cm) and Seedling Fresh Weight (mg) of Sesame Cultivars (GT-10 and JTS-8) after 96 hrs. |
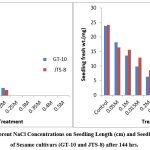 |
Figure 2: Effect of Different NaCl Concentrations on Seedling Length (cm) and Seedling Fresh Weight (mg) of Sesame Cultivars (GT-10 and JTS-8) after 144hrs. |
 |
Figure 3: Effect of Different Na2SO4 Concentration on Germination Percentage (%), Seedling Length (cm) and Seedling Fresh Weight (mg) of Sesame Cultivars (GT-10 and JTS-8) after 96 hrs. |
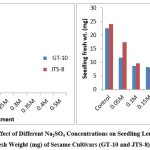 |
Figure 4: Effect of Different Na2SO4 Concentrations on Seedling Length (cm) and Seedling Fresh Weight (mg) of Sesame Cultivars (GT-10 and JTS-8) after 144 hrs. |
The behaviour of sorghum genotypes to different salt levels revealed that salinity was adverse to plant growth and development22. The germination of oat plant seeds and vegetative growth were substantially inhibited by increased salinity23.In addition to the harmful effects of specific ions, it is also considered that increased salt concentrations lowered the water potential in media, affecting germination efficiency by preventing seeds from absorbing water. It is assumed that overall seed germination would decrease with a reduction in the water transport into seeds during water uptake. Osmotic potential caused by salinity stress might inhibit the water uptake by the root or seed, followed by poor seed germination. Osmotic stress or sodium ions toxicity may be responsible for the salt-induced reduction of seed germination. The results showed a substantial negative linkage between seed germination %, seed germination time, and salinity level.
Conclusion
Overall, it was possible to conclude that both Sesamum indicum L. cultivars developed satisfactorily under the control conditions. However, when salinity stress (NaCl and Na2SO4) was increased, seedling growth and development decreased, as did both varieties’ germination percentages. The findings of this investigation show that beyond the concentration of 0.2 M NaCl, both seed germination percentage and seedling development are entirely inhibited. The JTS-8 variety was tolerable under various NaCl concentrations; however, GT-10 was more sensitive under various Na2SO4 concentrations.
Acknowledgement
The author1 would like to express their gratitude to the Head, Department of Botany, T.N.B College, Bhagalpur, T. M Bhagalpur University Bhagalpur, and Dr Ravi Ranjan Kumar and Dr Sima Sinha, Department of Plant Breeding and Genetic, Bihar Agricultural University, Sabour, Bhagalpur for providing laboratory facilities and sesame seeds, respectively.
Conflict of Interest
There is no conflict of interest among the authors.
References
- Hampson C.R., Simpson G.M. Effect of temperature, salt and osmotic potentials on early growth of wheat Germination. J. Bot, 1990; 68 (1): 524-525.
CrossRef - Gharsallah C., Fakhfakh , Gorsane F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB PLANTS, 2016; 8:plw055.
CrossRef - Hussain, S., Shaukat, M., Ashraf, M., Zhu, C., Jin, Q., Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. Change Agric. 2019; 13: 201–655.
CrossRef - Hasanuzzaman M., Nahar K., Fujita M. Ecophysiology and responses of plants under salt stress. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. Ecophysiology and responses of plants under salt stress. Springer, New York, NY, 2013; 25-87.
CrossRef - Flowers T. J., Flowers S. A. Why does salinity pose such a difficult problem for plant breeders? Water Manag, 2005; 78(1): 15-24.
CrossRef - Weiss, E.A. Oilseed Crops. 2nd Edition, Blackwell Science, Malden, 2000; 259-273.
- Kannan B., Ramamoorthi N., Paramsivam K. Influence of salt stress on seedling vigour in cowpea [Vigna unguiculata (L.) Walp] Sci. Digest, 2002; 22 (4): 293-294.
- Anuradha S., Rao S.S.R. Alleviating influence of Brassinolide on salinity stress induced inhibition of germination and seedling growth of rice. Indian J. Plant Physiol, 2002;7(4 N.S):384-387.
- Kao W.Y., Tsai T.T., Tsai H.C. Response of three Glycine species to salt stress. Environ Exp Bot, 2002; 56:120–125.
CrossRef - Demir I., Mavi K., Ozcoban M., Okcu G. Effect of salt stress on germination and seedling growth in serially harvested aubergine (Solanum melongena ) seeds during development. Israel. J. Plant Sci, 2003; 51: 125-131.
CrossRef - Jamil, M., Lee, D. B., Jung, K. Y., Ashraf, M., Niazi, S. L. B., Arshad, A., Mahmood, I. A., Rha, E. Effect of salt (NaCl) stress Growth and ionic relations of Brassica campestris on germination and early seedling growth of and B. juncea (L.) Czern & Coss. under induced salt four vegetables species. Cent. Eur. Agric., stress. Pakistan Journal of Agricultural Sciences, 2006 ; 7(2) :273-282.
- Duan D.Y., Li W.Q., Liu X.J., Ouyang H., An P., Duan D.Y. Seed germination and seedling growth of Suaeda salsa under salt stress. Bot. Fennici, 2007; 44: 161-169.
- Kandil A.A., Sharief A.E., Ahmed K.H.R. Performance of some soybean Glycine max Merrill. Cultivars under salinity stress to germination characters. IJAAR, 2015; 6(3): 48-56.
- Podder S., Ray J., Das D., Sarkar B.C. Effect of salinity (NaCl) on mungbean germination and seedling growth (Vigna radiata ), J. Biosci. Agric. Res. 2020; 24(02): 2012-2019.
CrossRef - Xue Z. Y., Zhi D. Y., Xue P. Enhanced salt tolerance of transgenic wheat (Triticumae sativum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci .2004; 167: 849-859.
CrossRef - Hakim M. A., Juraimi A. S., Begum M., Hanif M. M., Ismail M.R., Sadamat A. Effect of salt stress on germination and early seedling growth of rice. J. Biotech, 2010; 9 (13): 1911-1918.
CrossRef - Foolad M.R., Jones R.A. Mapping salt-tolerance genes in tomato using trait-based marker analysis. Appl. Genet, 1993; 87:184 – 192.
CrossRef - Huang J., Reddman R.E. Salt tolerance of Hordeum and Brassica species during germination and early seedling growth. Jan. Sci, 1995; 75:85-819.
CrossRef - Moud, A. M., Maghsoudi, K. Salt stress effects on respiration and growth of germinated seeds of different wheat cultivars. J. Agric. Sci, 2008; 4(3): 351-358.
- Jennette S., Jimenez B., Craig R., Lynch J.P. Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop Sci, 2002; 42: 1584-1594.
CrossRef - Jahan, I., Khan, D., Zaki, M. J. Effects of NaCl and Na2SO4 salinization on germination and early seedling growth of fifteen germplasms of Guar (Cyamopsis tetragonoloba (L.)Taub.)–In Vitro. Int .J. Biol. Biotech, 2020; 16(2):533-589.
- Rajabi Dehnavi, A., Zahedi, M., Ludwiczak, A., Cardenas Perez, S., Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agron, 2020; 10(6): 859.
CrossRef - Islam M.M., Al-Mamun S.S., Islam S.T. Impact of different levels of NaCl induced salinity on seed germination and plant growth of fodder oats (Avena sativa). J. Bangladesh Agric. Univ. 2022; 20(1): 40-48.
CrossRef

