Cervical Vertebral Bone Biopsy: Challenges and Tricks
ORIGINAL ARTICLE
Cervical Vertebral Bone Biopsy: Challenges and Tricks
PK Chinniah1, B Gopal2, V Moses1, SN Keshava1
1 Department of Radiology, Christian Medical College Hospital, Vellore, India
2 Katpadi Scan Centre, Katpadi, India
Correspondence: Prof SN Keshava, Department of Radiology, Christian Medical College Hospital, Vellore, India. Email: aparna_shyam@yahoo.com
Submitted: 11 Jun 2021; Accepted: 3 Sep 2021.
Contributors: SNK and VM designed the study. PKC and BG acquired the data, analysed the data and drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Institutional Review Board of Christian Medical College, Vellore, India (IRB No.: 13125).
Informed consent was waived by the Institutional Review Board because of the retrospective nature of this study.
Abstract
Objective
Biopsies of cervical spinal lesions are often challenging procedures with significant risk of complications.
Although computed tomography (CT)–guided biopsy of the thoracic and lumbar spine is considered a safe and
accurate procedure, cervical spine biopsies are less commonly performed. The aim of this retrospective study was
to evaluate the diagnostic accuracy of CT-guided needle biopsies for lesions of the cervical spine.
Methods
Results of 27 CT-guided biopsies of cervical spine lesions performed between February 2000 and May
2020 in a tertiary care teaching institute in India were retrieved and analysed.
Results
An adequate diagnostic yield was obtained in all 27 cases (100%). There were no major complications.
The common pathologies, approaches to the lesions, and the method of biopsy were studied.
Conclusion
CT-guided biopsy of the cervical spine with appropriate case selection and planning is a safe procedure
with high diagnostic yield.
Key Words: Retrospective Studies; Tertiary Healthcare; Biopsy, Needle; Tomography, X-Ray Computed; Cervical Vertebrae
中文摘要
頸椎骨活檢:挑戰與技巧
PK Chinniah、B Gopal、V Moses、SN Keshava
目的
頸椎病變活檢常是具有挑戰性的操作,具有較高的併發症風險。儘管CT引導下的胸椎和腰椎活檢被視為是一種安全且準確的操作,但頸椎活檢並不常見。本回顧性研究的目的在於評估 CT 引導下針刺活檢對頸椎病變的診斷準確性。
方法
分析 2000 年 2 月至 2020 年 5 月間在印度一所三級教學醫療機構完成的 27 例 CT 引導下頸椎病變活檢的結果。
結果
全部27名患者 (100%) 都獲得了足夠的診斷率。沒有重大併發症。本文描述常見的病理、病變的活檢路徑和活檢方法。
結論
在適當的病例選擇和規劃的前提下,CT引導下頸椎活檢是一種安全、診斷率高的手術。
INTRODUCTION
Biopsies of cervical spine are challenging procedures
due to the relatively small size of the cervical vertebrae,
limited access, difficult approach, and proximity to vital
organs. The present study addresses the case selection,
different approaches and techniques of computed
tomography (CT)–guided biopsy of cervical spinal
lesions to evaluate the diagnostic yield of this approach.
METHODS
In this study, data of 27 cases of percutaneous cervical
spine biopsy performed in a tertiary care hospital
between February 2000 and May 2020 were collected
retrospectively from the PACS (picture archive and
communication system) [GE Centricity 3.2; GE
Healthcare IT, Barrington [IL], US] and analysed.
Preprocedural evaluation included plain radiography, CT
or magnetic resonance imaging of the cervical spine, and
bone scan. Spine lesions with large exophytic soft tissue
components were not included, as soft tissue biopsy
was performed in those cases. Biopsies were performed
after a coagulation profile, including prothrombin time,
activated partial thromboplastin time, and platelet count.
All the patients in the study had normal coagulation
profile. Most of the biopsies were performed in an
outpatient setting.
The approach of biopsy was decided based on the location
of the lesion, the proximity of the lesion to the critical
structures of the neck (such as neurovascular bundles),
and the patient’s comfort. Some of these patients may
be on a supportive neck collar. Care must be taken while
removing the neck collar to avoid inadvertent injury to
the spinal cord.
A radiopaque guidewire was used to plan and mark
the needle entry site in patient’s skin, following which
local area was painted with 10% povidone iodine for
3 times (approximately for 30 seconds) and 1%
lignocaine was administered as local anaesthesia.
Usually, the procedures are done under local anaesthesia
for adults, while general anaesthesia is required for children and may be required sometimes for adults.
Partial pressure of oxygen and blood pressure were
continuously monitored during the procedures. While
advancing the needle, CT in the region of interest and
ultrasound usage will ensure safe trajectory. All spinal
biopsies were done under CT guidance. Dedicated CT
scanners (Siemens Somatom, Erlangen, Germany and
Philips Brilliance, Amsterdam, Netherlands) for biopsy
equipped with patient-monitoring devices were used.
Biopsy samples were sent for histopathology and culture
analysis.
Anterior / Lateral Approach for Vertebral
Body or Prevertebral Lesions
Coaxial Graded Needle Exchange Technique
Anterior approach is usually used for vertebral body
lesions. Needle direction is planned on CT. A 22-gauge
lumbar puncture needle or Chiba needle (Cook Medical,
Bloomington [IN], US) is first passed along the expected
direction of the target. Ultrasound guidance is used to
avoid major vascular structures such as carotid artery and
internal jugular vein. The direction of the needle and its
tip on the bone surface is confirmed on CT. The stylet is
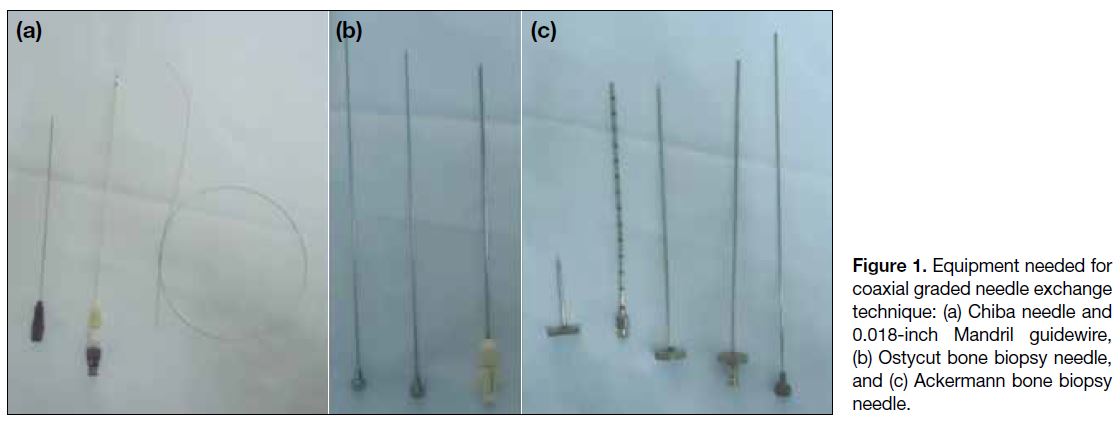
removed and the stiff end of a short 0.018-inch Mandril
guidewire (Cook Medical, Bloomington [IN], US;
Figure 1a) is passed into the lumen of the Chiba needle
for a distance approximating the length of the stylet, till it
touches the bone. The Chiba needle is removed over the
wire and the tract is dilated by a catheter (Neffset; Cook
Medical, Bloomington [IN], US), which is withdrawn
and then reinserted along with a wide-bore bone biopsy
cannula (Ostycut bone biopsy needle [Figure 1b] or
Ackermann bone biopsy needle [Figure 1c]; Cook
Medical, Bloomington [IN], US) over the guidewire until
it again touches the bone. After confirming the direction
on CT, the catheter and the guidewire are replaced by a
bone biopsy needle inserted into the cannula and biopsy
is performed (Figures 2 and 3). The position of the
biopsy needle is confirmed on CT. Adequate numbers of
samples are collected for histopathology and culture. A
final CT scan is performed after removal of the needle to
assess for haemorrhage.
Figure 1. Equipment needed for
coaxial graded needle exchange
technique: (a) Chiba needle and
0.018-inch Mandril guidewire,
(b) Ostycut bone biopsy needle,
and (c) Ackermann bone biopsy
needle.
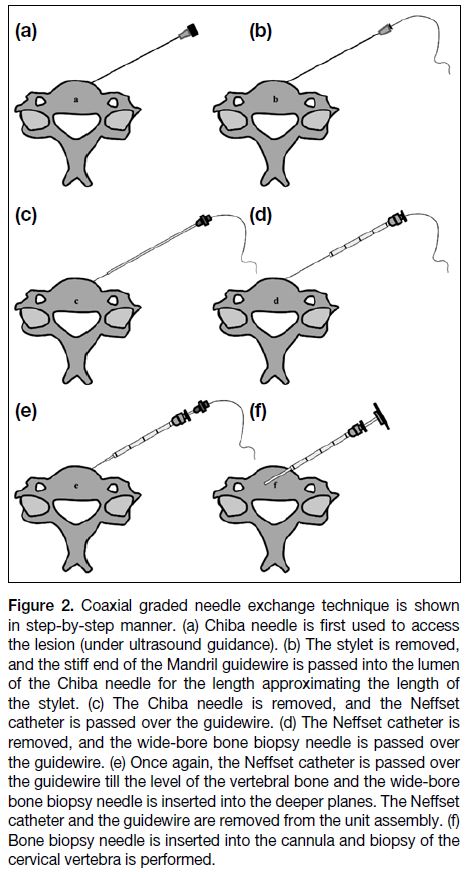
Figure 2. Coaxial graded needle exchange technique is shown
in step-by-step manner. (a) Chiba needle is first used to access
the lesion (under ultrasound guidance). (b) The stylet is removed,
and the stiff end of the Mandril guidewire is passed into the lumen
of the Chiba needle for the length approximating the length of
the stylet. (c) The Chiba needle is removed, and the Neffset
catheter is passed over the guidewire. (d) The Neffset catheter is
removed, and the wide-bore bone biopsy needle is passed over
the guidewire. (e) Once again, the Neffset catheter is passed over
the guidewire till the level of the vertebral bone and the wide-bore
bone biopsy needle is inserted into the deeper planes. The Neffset
catheter and the guidewire are removed from the unit assembly. (f)
Bone biopsy needle is inserted into the cannula and biopsy of the
cervical vertebra is performed.
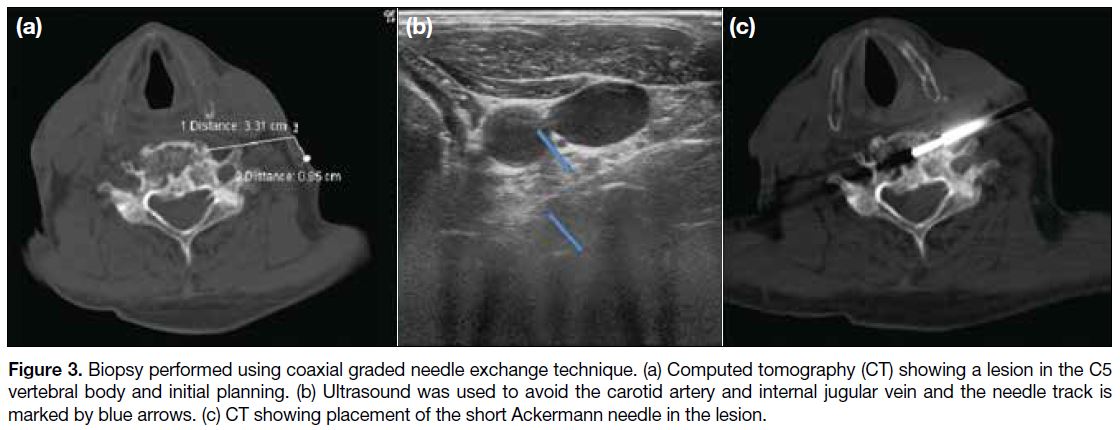
Figure 3. Biopsy performed using coaxial graded needle exchange technique. (a) Computed tomography (CT) showing a lesion in the C5
vertebral body and initial planning. (b) Ultrasound was used to avoid the carotid artery and internal jugular vein and the needle track is
marked by blue arrows. (c) CT showing placement of the short Ackermann needle in the lesion.
The coaxial graded needle exchange technique may be
used for other approaches, as well as with other similar
suitable combinations of the needles.
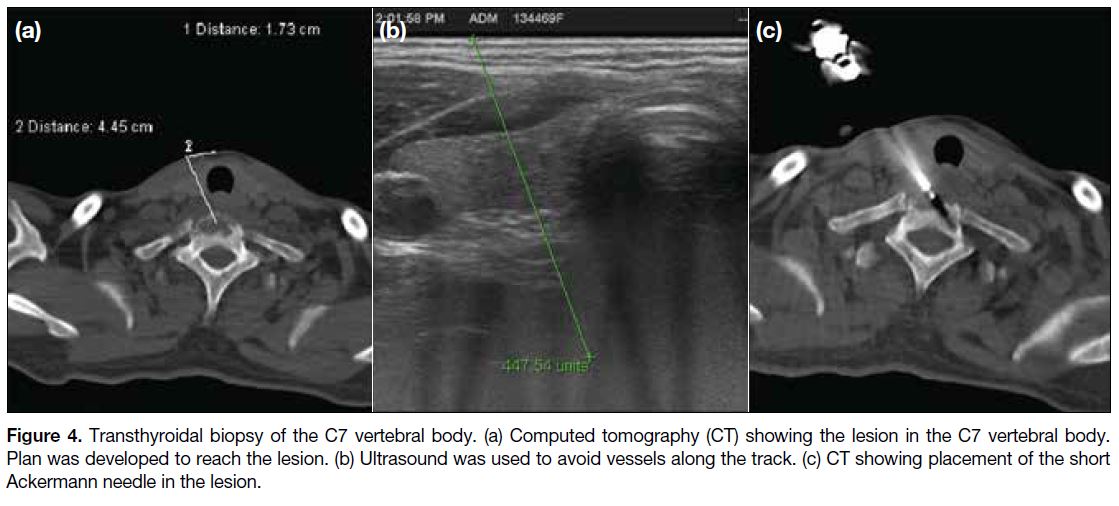
Transthyroid Technique
This is done keeping the patient in supine position.
Traversing of the needle through the thyroid is done with
ultrasound guidance to avoid vessels along the track
(Figure 4). Ultrasound offers real-time visualisation
of the great vessels of the neck and to plan the needle
trajectory safely.
Figure 4. Transthyroidal biopsy of the C7 vertebral body. (a) Computed tomography (CT) showing the lesion in the C7 vertebral body.
Plan was developed to reach the lesion. (b) Ultrasound was used to avoid vessels along the track. (c) CT showing placement of the short
Ackermann needle in the lesion.
Posterior Approach for Posterior Element
Lesions
The posterior approach is performed similar to biopsies
of the thoracolumbar spine (Figure 5). The patient is
positioned either prone or in lateral decubitus position
for the purpose of stabilising the cervical spine. After
localising the lesion, local anaesthesia is injected. Biopsy
of the lesion or soft tissue is done using a coaxial system.
Pillows are used below the head and neck to support the
cervical spine and provide comfort to the patient.
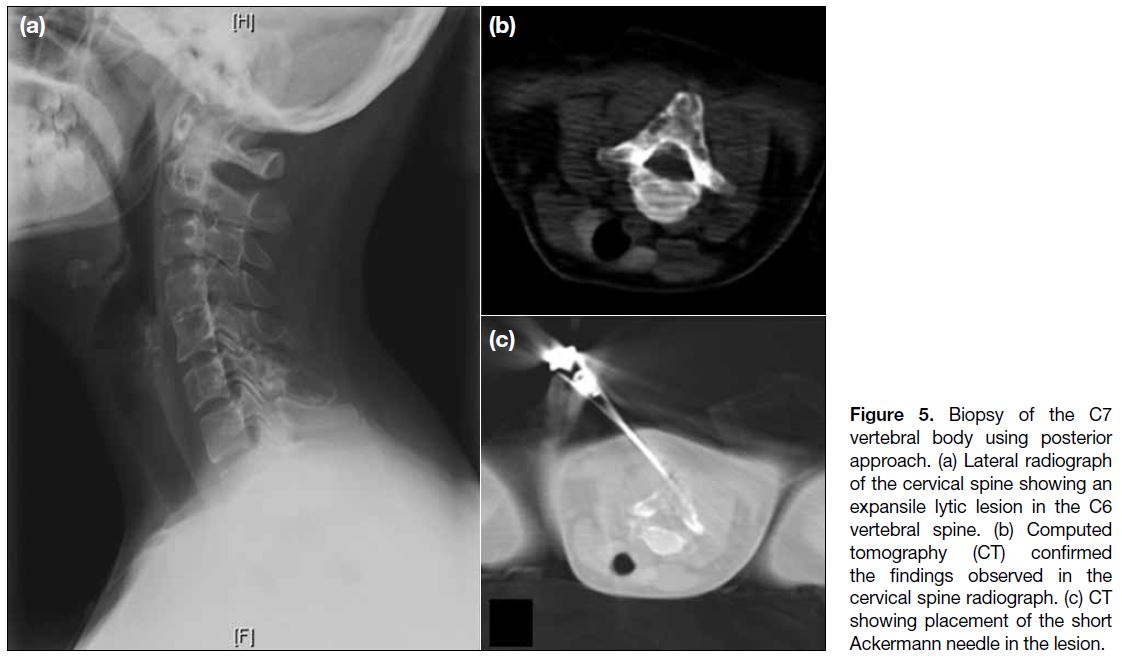
Figure 5. Biopsy of the C7
vertebral body using posterior
approach. (a) Lateral radiograph
of the cervical spine showing an
expansile lytic lesion in the C6
vertebral spine. (b) Computed
tomography (CT) confirmed
the findings observed in the
cervical spine radiograph. (c) CT
showing placement of the short
Ackermann needle in the lesion.
Transoral Approach for C1 or C2 Lesions
The transoral approach can be attempted for lesions in
the atlanto-axial region and is performed under general
anaesthesia. After retracting the soft palate and uvula,
a bone biopsy needle is slowly advanced through the
posterior pharyngeal wall and further into the underlying
C1 or C2 vertebra (Figure 6).
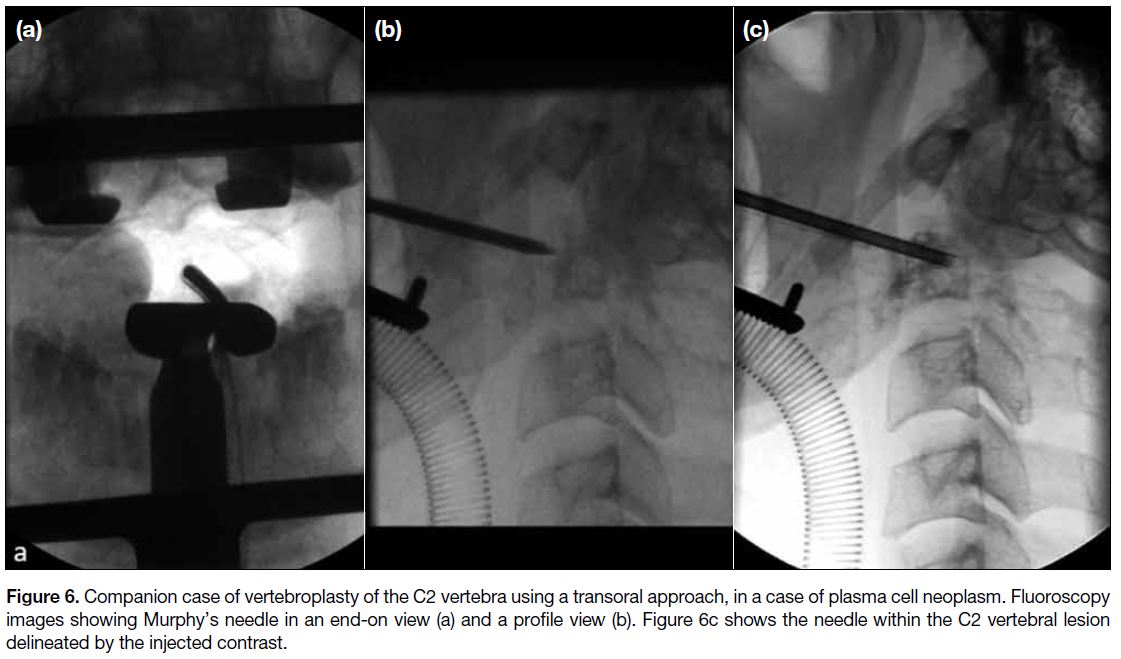
Figure 6. Companion case of vertebroplasty of the C2 vertebra using a transoral approach, in a case of plasma cell neoplasm. Fluoroscopy
images showing Murphy’s needle in an end-on view (a) and a profile view (b). Figure 6c shows the needle within the C2 vertebral lesion
delineated by the injected contrast.
RESULTS
The study population comprised 27 cases that included
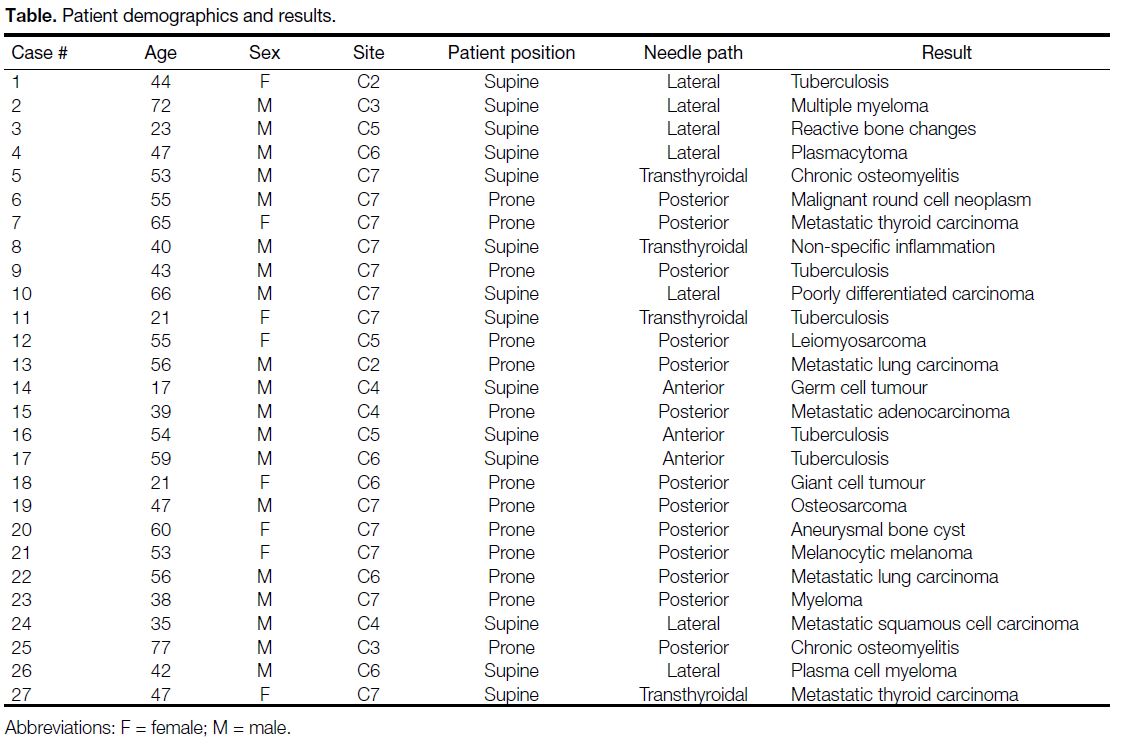
19 males and 8 females with age ranging from 17 to 77 years. A summary of the cases and the techniques
used is given in the Table. The diagnostic yield was
considered adequate if the material was enough for
histopathological interpretation or there was a positive
microbial growth on culture. Such an adequate diagnostic
yield was possible in all the 27 cases (100%). Some
patients experienced mild discomfort and pain related
to the procedure, which was adequately controlled with
analgesics. There were no major complications.
Table. Patient demographics and results.
Proximity to vital structures such as the carotid artery, vertebral artery, and spinal cord is the challenge in
this location with respect to avoiding complications.
It is important to evaluate for complications even after considering all the precautions. Postprocedural CT,
clinical examination, and follow-up are vital.
DISCUSSION
Percutaneous spine biopsy was first described by
Ball in 1934. Image-guided biopsy was reported in
1949 with conventional radiographs, followed by
fluoroscopy in 1969, CT in 1981, magnetic resonance
imaging in 1986, and CT fluoroscopy in 1996. Initially,
open biopsies were performed, but percutaneous needle
biopsy offers a faster, more cost-effective approach
with fewer complications.[1] [2] [3] [4] Compared to surgical
biopsies, percutaneous needle biopsy is less invasive
and can usually be performed under local anaesthesia.
For cervical vertebral biopsy, different approach routes
are selected depending on position of the lesion.[5] [6]
The routes to access the cervical bone lesions in the
suprahyoid neck include transoral, sub mastoid, and
posterior approaches. Lesions in the infrahyoid neck
can be approached either medial or lateral to the carotid
sheath with the patient supine or via a posterior approach
with patient prone.
Compared to the much more commonly performed CT-guided
biopsy of the thoracic and lumbar spine, cervical
spine biopsies are considered more challenging. This is
because of the relatively smaller size of the vertebrae and
proximity to major vessels and spinal cord. In contrast
to the thoracolumbar spine, for cervical vertebral body
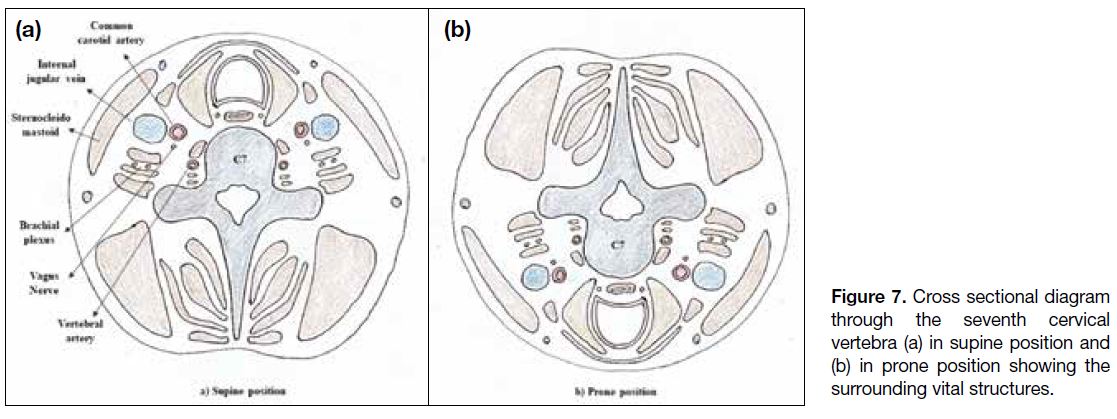
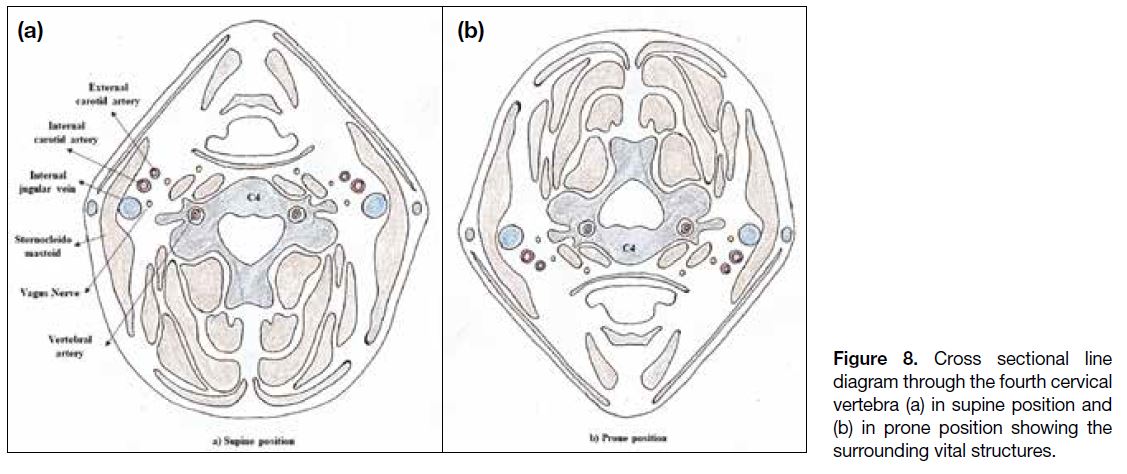
lesions, the patient needs to be positioned supine. Figures 7 and 8 show the vital structures surrounding the cervical
spine. The accuracy of the results is also lower in the
cervical spine compared to thoracolumbar spine.[7]
Figure 7. Cross sectional diagram
through the seventh cervical
vertebra (a) in supine position and
(b) in prone position showing the
surrounding vital structures.
Figure 8. Cross sectional line
diagram through the fourth cervical
vertebra (a) in supine position and
(b) in prone position showing the
surrounding vital structures.
Indications for imaging-guided spine biopsy include
confirming or excluding metastasis in a patient with a
known primary malignancy; excluding malignancy,
especially metastases or myeloma in vertebral body compression; and to confirm the diagnosis of infection
and to obtain sample of organism, e.g., spondylodiscitis
or osteomyelitis.[8]
There are no absolute contraindications for spine biopsy.[8]
Relative contraindications include bleeding disorder,
thrombocytopenia-platelet count (<50,000/mm3)
and inability of the patient to cooperate, which may
require general anaesthesia.
Complications include haematoma/active bleeding,
infection, neurological injury (e.g., cord compression),
pneumothorax, and tumour seeding along the needle
track if the lesion is a primary tumour (e.g., sarcoma).[8] [9]
Injury to vital structures can be minimised by real-time
ultrasound guidance during the initial course of needle
insertion to avoid major vessels, using a thinner needle
(21G) at the start of the procedure and exchanging for
a larger needle when avoidance of major structures
has been assured.[10] The cases did not have any major
complications.
The coaxial graded needle exchange technique described
ensures maximum safety by avoiding injury to major neurovascular structures. In our study, we were able to
achieve 100% results in attaining the diagnosis with no
major complications.
Wiesner et al[11] obtained sufficient sample in 96% (70/73)
cases and reported complications in 2/73 patients, in
which the patients became hypotensive during the
procedure. These patients were conservatively managed.
In one patient, the procedure was repeated after 2 days.
Cox et al[12] were able to get sufficient material for
histopathologic analysis in 41 out of 43 cases (95%).
Yang et al[13] observed a diagnostic yield of 80% in spinal tumours in 197 out of 247 patients including cervical
spine (23 lesions), thoracic spine (92 lesions), lumbar
spine (96 lesions), and sacral spine (36 lesions). Out of the 23 lesions in the cervical spine, four turned out to be
non-diagnostic. McKnight et al[14] were able to perform
CT-guided biopsies safely in endoscopically inaccessible
areas of head and neck.
Garg et al[15] were able to obtain core sample in all 122
cases (100%) in vertebral bone biopsy by using coaxial
needle biopsy and noted that core sampling provided
more specific diagnoses in 104/122 patients (85%).
Gala et al[16] found CT-guided percutaneous biopsy is
safer than open surgical biopsy with lower rates of
complications and shorter hospital stays.
In conclusion, CT-guided percutaneous biopsy of the
cervical spine is a minimally invasive, safe, and cost-effective procedure. Appropriate case selection and
planning are important. Additional ultrasound guidance
to avoid vessels and use of graded needle exchange
technique are helpful in certain cases to obtain adequate
diagnostic yield. The limitation of our study is its
retrospective nature.
REFERENCES
1. Yaffe D, Greenberg G, Leitner J, Gipstein R, Shapiro M, Bachar GN.
CT-guided percutaneous biopsy of thoracic and lumbar spine: a
new coaxial technique. AJNR Am J Neuroradiol. 2003;24:2111-3.
2. Tehranzadeh J, Tao C, Browning CA. Percutaneous needle biopsy
of the spine. Acta Radiol. 2007;48:860-8. Crossref
3. Adapon BD, Legada BD Jr, Lim EV, Silao JV Jr, Dalmacio-Cruz A.
CT-guided closed biopsy of the spine. J Comput Assist Tomogr.
1981;5:73-8. Crossref
4. Genant JW, Vandevenne JE, Bergman AG, Beaulieu CF, Kee ST,
Norbash AM, et al. Interventional musculoskeletal procedures
performed by using MR imaging guidance with a vertically open
MR unit: assessment of techniques and applicability. Radiology.
2002;223:127-36. Crossref
5. Gupta S, Henningsen JA, Wallace MJ, Madoff DC, Morello FA Jr,
Ahrar K, et al. Percutaneous biopsy of head and neck lesions
with CT guidance: various approaches and relevant anatomic and
technical considerations. Radiographics. 2007;27:371-90. Crossref
6. Kattapuram SV, Rosenthal DI. Percutaneous biopsy of the cervical
spine using CT guidance. AJR Am J Roentgenol. 1987;149:539-41. Crossref
7. Rimondi E, Staals EL, Errani C, Bianchi G, Casadei R, Alberghini
M, et al. Percutaneous CT-guided biopsy of the spine: results of
430 biopsies. Eur Spine J. 2008;17:975-81. Crossref
8. Peh W. CT-guided percutaneous biopsy of spinal lesions. Biomed
Imaging Interv J. 2006;2:e25. Crossref
9. Espinosa LA, Jamadar DA, Jacobson JA, DeMaeseneer MO,
Ebrahim FS, Sabb BJ, et al. CT-guided biopsy of bone: a
radiologist’s perspective. AJR Am J Roentgenol. 2008;190:W283-9. Crossref
10. Gupta S, Takhtani D, Gulati M, Khandelwal N, Gupta D,
Rajwanshi A, et al. Sonographically guided fine-needle aspiration
biopsy of lytic lesions of the spine: technique and indications. J
Clin Ultrasound. 1999;27:123-9. Crossref
11. Wiesner EL, Hillen TJ, Long J, Jennings JW. Percutaneous CTguided
biopsies of the cervical spine: technique, histopathologic
and microbiologic yield, and safety at a single academic institution.
AJNR Am J Neuroradiol. 2018;39:981-5. Crossref
12. Cox M, Pukenas B, Poplawski M, Bress A, Deely D, Flanders A.
CT-guided cervical bone biopsy in 43 patients: diagnostic yield
and safety at two large tertiary care hospitals. Acad Radiol.
2016;23:1372-5. Crossref
13. Yang SY, Oh E, Kwon JW, Kim HS. Percutaneous image-guided
spinal lesion biopsies: factors affecting higher diagnostic yield.
AJR Am J Roentgenol. 2018;211:1068-74. Crossref
14. McKnight CD, Glastonbury CM, Ibrahim M, Rivas-Rodriguez F,
Srinivasan A. Techniques and approaches for safe, high-yield CT-guided
suprahyoid head and neck biopsies. AJR Am J Roentgenol.
2017;208:76-83. Crossref
15. Garg V, Kosmas C, Josan ES, Partovi S, Bhojwani N, Fergus N, et al.
Computed tomography-guided percutaneous biopsy for vertebral
neoplasms: a department’s experience and hybrid biopsy technique
to improve yield. Neurosurg Focus. 2016;41:E17. Crossref
16. Gala KB, Shetty NS, Janu AK, Shetty N, Kulkarni SS. Percutaneous
CT guided vertebral biopsy: anatomy and technical considerations.
J Clin Interv Radiol ISVIR. 2021;5:150-7. Crossref