Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.
Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
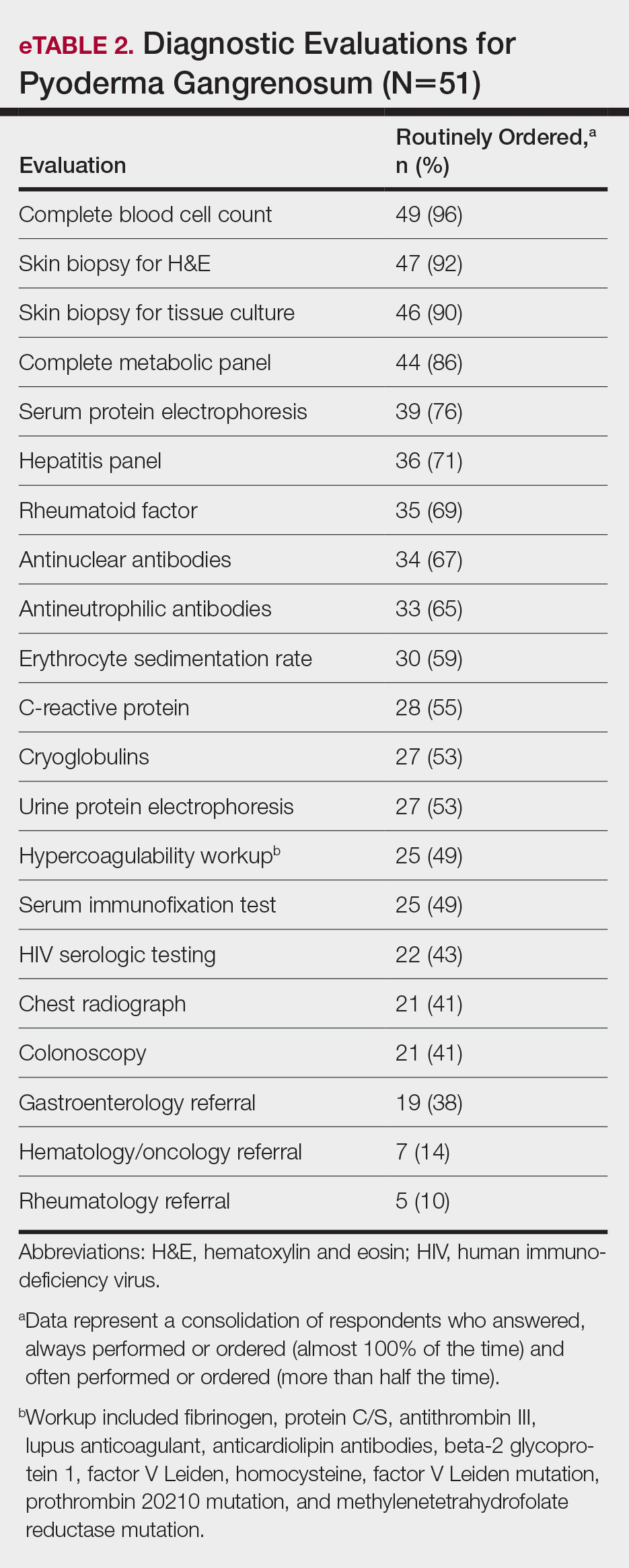
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).
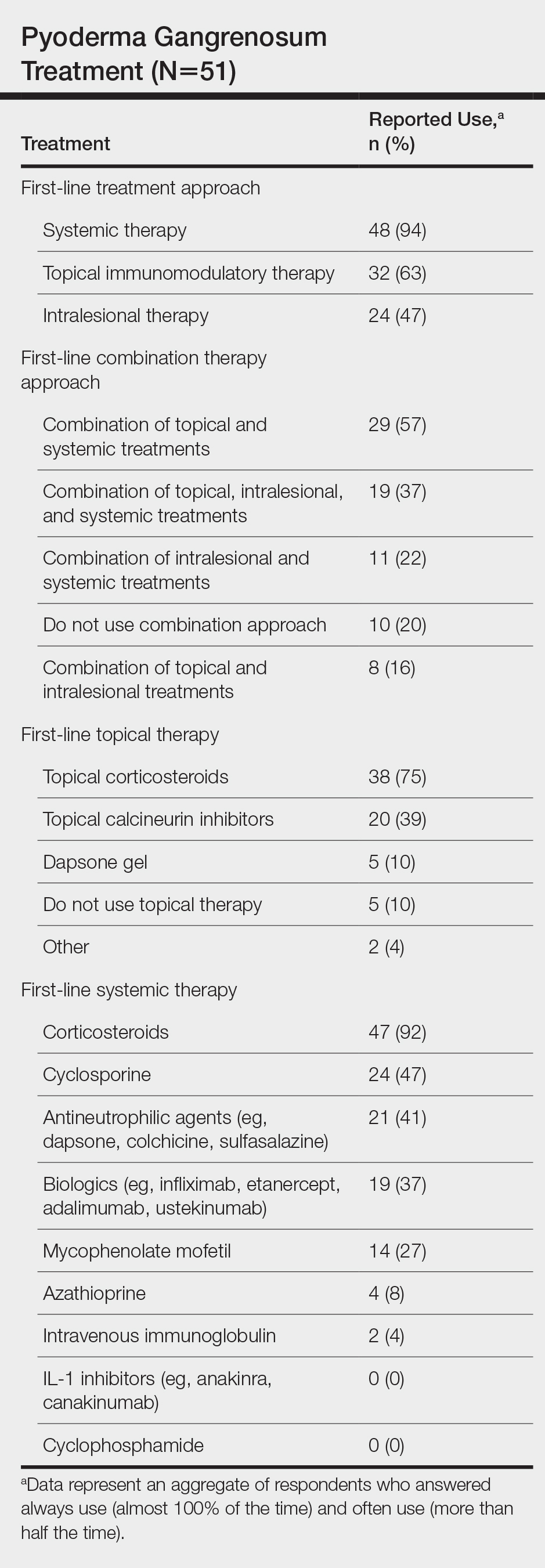
Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).
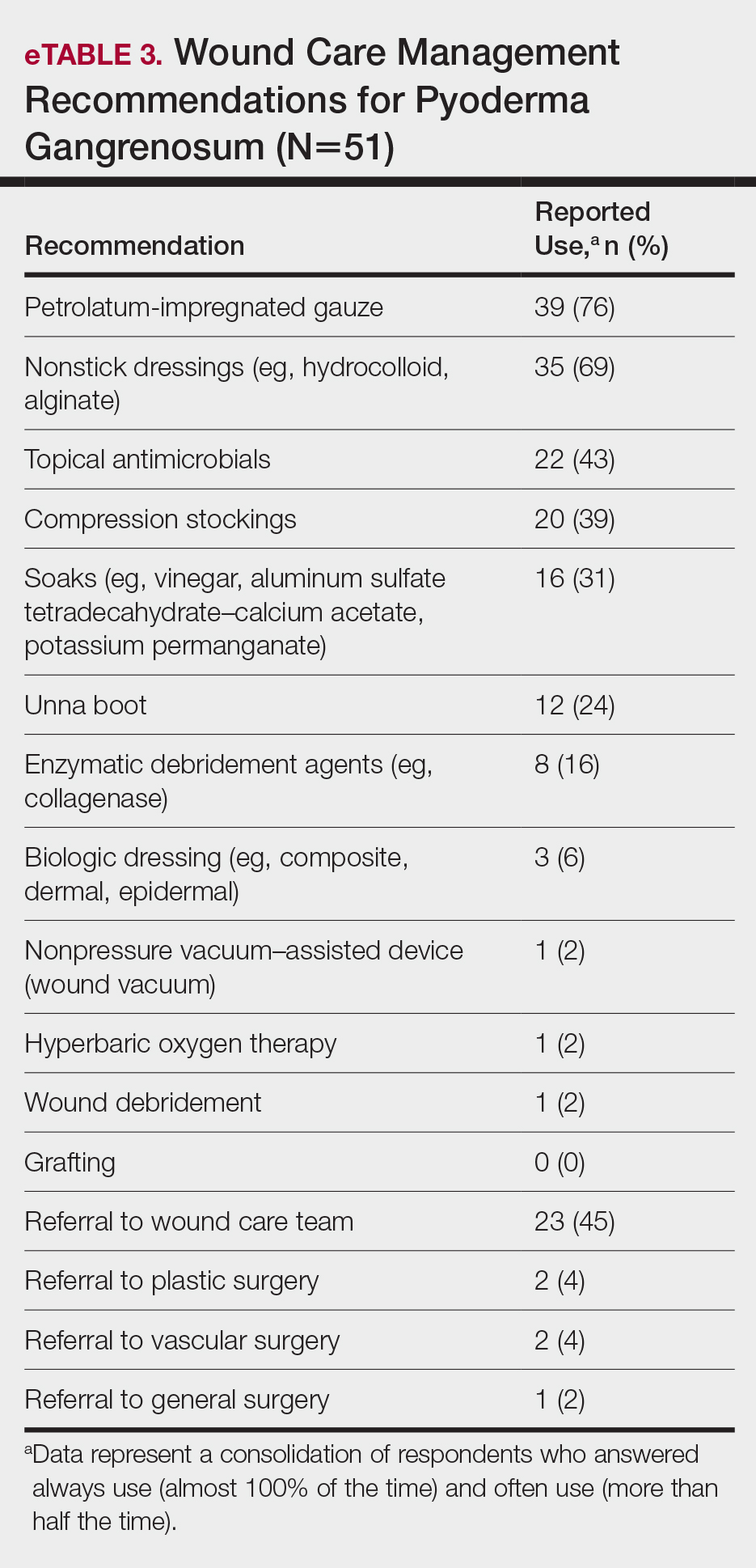
A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).