01 August 2023: Original Paper
Graft and Patient Survival in Kidney Transplant with Deceased Donor Using KDRI (Kidney Donor Risk Index), KDPI (Kidney Donor Profile Index), and EPTS (Estimated Post-Transplant Survival) in Colombia
Anabel Vanin A.DOI: 10.12659/AOT.940522
Ann Transplant 2023; 28:e940522
Abstract
BACKGROUND: EPTS (Estimated Post-Transplant Survival), KDRI (Kidney Donor Risk Index), and KDPI (Kidney Donor Profile Index) were developed aiming to ameliorate donor–recipient longevity matching in kidney transplants. They are based on a prediction model made using the United States population; evidence of their use outside EEUU remains limited. The aim of this study was to describe the quality of deceased-donor kidneys and to determine recipient and graft survival, glomerular filtration rate, and incidence of delayed graft function in renal transplantation according to these indices in Cali, Colombia.
MATERIAL AND METHODS: In this historical cohort study, Kaplan-Meier method was used to analyze survival of recipient and graft according to the values of the indices categorized by quintiles. Glomerular filtration rate and incidence of delayed graft function were also analyzed according to KDRI and KDPI.
RESULTS: We included 380 patients. Medians of EPTS, KDRI, and KDPI were 24% (IQR 9-60), 0.8 (IQR 0.71-0.99), and 27% (IQR 13-49), respectively. Two-year survival was 97.8% in recipients with EPTS ≤20% and it decreased with higher values of the index. Recipient and graft survival were lower for all periods when donors had KDPI >80%. Incidence of delayed graft function was higher in patients whose donors had KDPI ≥60% (44% vs 21%). Glomerular filtration rate decreased with the highest values of KDPI for all periods.
CONCLUSIONS: Our study represents the initial evaluation of the usefulness of these indices in Colombia. Our results suggest that KDRI, KDPI, and EPTS may serve as valuable tools for kidney allocation in our setting. Further research with larger sample sizes is necessary to validate these indices in our population.
Keywords: Kidney Transplantation, Graft Survival, renal insufficiency, Glomerular Filtration Rate, Humans, United States, Delayed Graft Function, Cohort Studies, Colombia, Tissue Donors, Kidney, Retrospective Studies
Background
One of the dilemmas in renal transplantation is the allocation of a kidney to a recipient with a given chance of survival according to its quality. To facilitate this process, several indices have been created to measure graft quality and estimate graft duration. In 2002, the concepts of the Expanded Criteria Donor (ECD) and Standard Criteria Donor (SCD) were created [1]. Subsequently, indices with better predictive capacity were developed: the Kidney Donor Risk Index (KDRI) and Kidney Donor Profile Index (KDPI) [2,3]. The KDRI is based on a prediction model that assigns continuous risk score to deceased donor kidneys according to the donor and graft characteristics. A variant of the KDRI, known as the KDPI, is a useful tool for deciding whether or not to accept an offer of a deceased donor kidney. The KDPI removes transplant-related factors from the KDRI, and is normalized to a percentile score (eg, risk of a kidney with a KDPI of 70 is judged to be worse than risk of 70% of kidneys recovered for transplantation in the prior calendar year). The Estimated Post-Transplant Survival (EPTS) index is a score that combines 4 clinical parameters (age, time on dialysis, prior solid organ transplant, and diabetes) to estimate post-transplant survival of kidney transplant recipients. The allocation rules in the United States use the KDPI for donors and the EPTS score for longevity matching between a portion of donors and recipients. Those donor kidneys with KDPI 20% or less will first be offered to adult candidates with the top 20% of EPTS scores, followed by candidates with EPTS outside the top 20% [2,3]. Although the reference population for these prediction models is exclusively from North America, their use has expanded worldwide, and validation studies have been conducted in several countries [4–6]. In Latin America, few studies have measured the quality of the transplanted kidneys using these indices. This study aimed to evaluate the quality of deceased donor organs used in 2 centers in Cali, Colombia, and to determine patient and graft function and survival in renal transplantation according to the KDRI, KDPI, and EPTS indices.
Material and Methods
DESIGN:
This was a historical cohort study of renal transplant recipients in 2 high-complexity institutions. This study was reviewed and approved by the Ethics Committee of both institutions.
SETTING:
This study was performed using patient data from 2 high-complexity institutions in Cali, Colombia, between March 2010 and November 2017.
POPULATION:
All patients aged >18 years who underwent deceased donor kidney transplant between March 2010 and November 2017 at either institution, with at least 1 month of post-transplant follow-up, were included.
SAMPLING:
All patients with at least 1 month of follow-up were included; no calculation of the sample size or sampling procedure was performed.
DATA COLLECTION:
Information was collected from medical records in paper format designed for this purpose. Questionnaires were completed by transplant nurses, transplant surgeons, and nephrologists. The researcher registered the de-identified data in a password-protected database.
INDICES AND DEFINITIONS:
Organ allocation was originally performed using the ECD and SCD. The KDRI, KDPI, and EPTS indices were retrospectively calculated for this study using the calculator available on the Organ Procurement and Transplantation Network OPTN website [7–9]. The definitions of primary non-function (PNF), good graft function (GGF), delayed graft function (DGF), or slow graft function (SGF) were based on the articles by El-Zoghby et al [10] and Humar et al [11]. Graft loss was defined as return to dialysis, retransplantation, or death. To estimate graft function, the post-transplant glomerular filtration rate was calculated using the CKD-EPI formula at months 1, 3, 6, 12, and 24.
DATA ANALYSIS:
Categorical variables were described as proportions. The distribution of continuous variables was evaluated using the Shapiro–Wilk test and expressed as medians or means with their respective interquartile ranges (IQRs) or standard deviations (SDs). Patient and graft survival functions were calculated using the Kaplan-Meier method according to the groups defined for the EPTS, KDRI, and KDPI indices. A log-rank test was performed if proportional hazard assumptions were met.
The KDRI and KDPI values were classified into quintiles (0–20%, 21–40%, 41–60%, 61–80%, and 81–100%), and the cumulative incidence of DGF was compared among the groups. Finally, the median of the glomerular filtration rate was compared among the quintiles for each month using the Kruskal-Wallis test. A P value of <0.05 was considered statistically significant.
Results
EPTS, KDRI, AND KDPI:
The median EPTS score in recipients was 24% (IQR, 9–60). A total of 166 (43.7%) patients had an EPTS score between 0% and 20%, whereas 54 (14.2%) had an EPTS score between 80% and 100% (Table 1).
The median KDRI score was 0.8 (IQR, 0.71–0.99), with a minimum value of 0.56 and maximum of 1.79.
The median KDPI score was 27% (IQR, 13–49). Slightly more than one-third of the donors had a KDPI score between 0% and 20%, and only 21 (5.5%) had a KDPI score between 81% and 100% (Table 2).
Figures 1 and 2 show the distribution of patients with SCD and ECD in the different KDRI and KDPI ranges, respectively. Donor-labeled ECDs were distributed in the KDRI >1.0 and KDPI >60% groups.
Figure 3 shows how the median EPTS score increased in the higher KDPI categories. This difference was not as clear in the 0–20% and 20–40% categories, probably because of the variability in EPTS values in these groups.
PATIENT SURVIVAL:
The 2-year patient survival function rates (Figure 4) were 97.8% in recipients with EPTS scores between 0% and 20%, 95.9% in those with EPTS scores between 21% and 40%, 97.6% in those with EPTS scores of 41–60%, 80.6% in those with EPTS scores between 61% and 80%, and 82.1% in those with EPTS scores between 81% and 100%. The 2-year survival tended to be lower in patients with EPTS scores of >60%.
Figure 5A and 5B show patient survival according to the KDRI quintiles and KDPI ranges respectively. The 2-year survival rates were 97.6% in recipients with KDPI scores of 0–20%, 98.1% in those with KDPI scores of 21–40%, 93.7% in those with KDPI scores of 41–60%, 86.1% in those with KDPI scores of 61–80%, and 55.5% in those with KDPI scores of 81–100%. Survival was lower in all periods analyzed in patients who received kidneys with KDPI scores of >80%. The log-rank test was not performed because the results did not meet the proportional hazard assumptions.
GRAFT SURVIVAL:
Graft survival was lower in patients who received transplants from donors with KDRI scores of >1.05 or KDPI scores of >80% (Figure 6A, 6B). No retransplantation occurred. The 2-year survival function rates were 95.0% in those with KDPI scores of 0–20%, 91.0% in those with KDPI scores of 21–40%, 85.2% in those with KDPI scores of 41–60%, 82.7% in those with KDPI scores of 61–80%, and 55.5% in those with KDPI scores of 81–100%. A log-rank test was not performed because the results did not meet the proportional hazard assumptions.
GRAFT FUNCTION:
The incidence of DGF was significantly higher in patients whose donors had KDRI scores of >1.06 (15% vs 33%, p<0.001) or KDPI scores of >60% (21% vs 44%, p<0.001).
The glomerular filtration rate significantly decreased as the KDRI and KDPI scores increased in all periods evaluated. Figure 7A and 7B show the change in the glomerular filtration rate for month 12 according to the KDRI and KDPI, respectively.
Discussion
The KDRI and KDPI were developed in a population of 69 440 adult deceased donor renal transplant recipients reported to the US Scientific Registry of Transplant Recipients between 1995 and 2005 [2]. These indices have been validated in different countries [3–6,12–20]. To date, no study has used these indices in Colombia or Latin America.
Our results showed that donors previously classified as having ECD were distributed among those with KDRI scores of >1.0, a finding similar to that reported by Rao [2]. Regarding the KDPI, donors with expanded criteria appeared in the KDPI groups of 61–80% and >80%. We may have labeled donors who were not at increased risk of graft loss as having ECD. On the other hand, 19% of donors with KDPI scores of 81–100% corresponded to SCD, probably because the KDRI and KDPI take into account more variables, resulting in the inclusion of the kidneys that would have been labeled as unsuitable for transplantation in the previous classification.
Moreover, the increase in EPTS scores at higher KDPI values is consistent with the rationale of the organ allocation system. In our study, patient survival was lower in recipients with EPTS scores of >60%. It is striking that most recipients had EPTS scores of ≤20%, which is different from the data reported in the US and Australian series, where the risk profile of patients appears to be higher than that of our recipients.
There was a higher frequency of graft loss in patients who received transplants from donors with KDRI scores of >1.05 or KDPI scores of >80%. Graft survival in patients with KDPI scores of ≤60% compares favorably with the estimated 1-year graft survival data for different KDPI values in the United States in 2016 [8] but is lower in the 61–80% KDPI group (89.2% vs 90.1%), and the difference was more marked in the 81–100% KDPI group (66.6% vs 82.9%).
An analysis of United Network for Organ Sharing (UNOS) data comparing the survival of patients aged >60 years transplanted with the kidneys with KDPI scores of >85% versus those remaining on the waiting list showed a significant reduction in mortality after the first year in transplanted patients [17]. It has been previously shown that the survival advantage of kidney transplantation with elevated KDPI scores is greater in patients aged >50 years [3].
The median age of the donors was 29 years (IQR, 19–46), and the oldest donor was 63 years old. This contrasts with European data, as in a Dutch study in which this index was validated [4], the median age of donors was 55 years (IQR, 40–60), and in a Spanish study [19] the mean age of donors was 63 years (SD, 8.2). The younger age of our donors partly explains the differences found in the KDRI scores when compared with those in previous studies. In the present study, the median KDRI score was 0.8. This value is lower than that reported by Rao et al in the original study in which the index was described, in which the median KDRI score was 1.05. In a report based on the Dutch Organ Transplant Registry [4], the median KDRI score was 1.21, whereas in a study conducted in Grenada [19], the mean KDRI score was 1.08. The maximum KDRI scores for donors were 1.79 in Colombia, 4.2 in the United States, and 3.0 in the Netherlands.
A similar behavior was observed for the KDPI values. The median KDPI score in our study was 27% (IQR, 13–49). A study of a group of patients in Berlin [20] reported a median KDPI score of 66% (IQR, 41–92), and in a Spanish study, the mean KDPI score was 86%. The proportion of patients who received transplants from donors with KDPI scores of 0–20% was 35.26% in our recipients, whereas in the United States in 2015, it was 22.69%, and 5.53% received a kidney with a KDPI score of 81–100% vs 7.9% in the American series [15,22].
The incidence of DGF increased as the donor KDRI and KDPI scores increased. There was a significant difference when this incidence was compared using the cutoff points of 1.06 and 60% for the KDRI and KDPI, respectively. These findings are similar to those reported in an analysis of 2161 patients in the United States, which found that the DGF was significantly lower in patients who received kidneys with KDPI scores of ≤60% than in those with score of 61–80% and 81–100% [22] The significant decrease in the glomerular filtration rate as the KDRI and KDPI scores increased in all time periods allows inferring its usefulness to predict graft survival. To the best of our knowledge, this has only been evaluated in a few studies [6].
The implementation of the EPTS and KDRI/KDPI as important, but not unique, elements of the decision tree in kidney allocation may lead to an increase in the number of transplants. An evaluation of the acceptance of kidney offers at a center linked to Eurotransplant [23] showed that the number of transplants increased by 26% once the KDRI started to be used. In contrast, the kidney discard rates in the United States before and after 2012 (when the KDPI began to be reported in kidney offers) did not show a significant change: 18.1% in the ECD era and 18.3% in the KDPI era [24]. However, its application in settings other than North America is limited by differences in organ harvesting strategies and clinical outcomes [25,26].
A limitation of this study is the sample size, which was not sufficient to perform a subgroup analysis of the lowest categories of indices or their validation. However, we note that at the time of writing this article, we found no published reports on the use of the EPTS, KDRI, and KDPI in patients undergoing deceased donor kidney transplantation in Colombia or Latin America and that the results of graft and recipient survival are similar to those found in other studies. Analysis of the data from this cohort of patients showed that the KDRI and KDPI scores of our donors were lower than those of donors reported in other countries, probably because the donors tended to be younger. It is also likely that the organs from older patients with satisfactory graft survival were not rescued. Although these indices should not be used as the only tools when deciding on organ allocation [27,28] as they do not consider all factors that may influence transplant outcomes, they certainly help achieve a more accurate measurement of donor quality and estimated recipient survival. Their application is easy, and the required data are available at the time of the offer. The fear of using less-than-ideal donors may decrease with the use of these tools [29], which could increase the number of people benefiting from kidney transplantation.
Conclusions
To the best of our knowledge, this is the first report to evaluate the quality of deceased donor kidneys in Colombia using these indices. Our findings provide evidence that favors their use as a tool to properly allocate the kidneys. Studies with larger sample sizes are required to validate these indices in the study population.
Figures
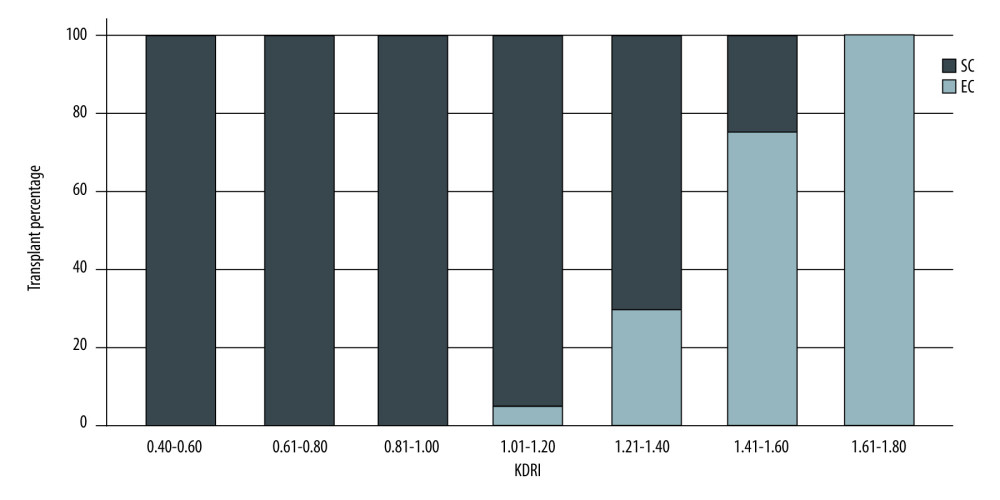 Figure 1. Donor classification according to KDRI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 1. Donor classification according to KDRI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. 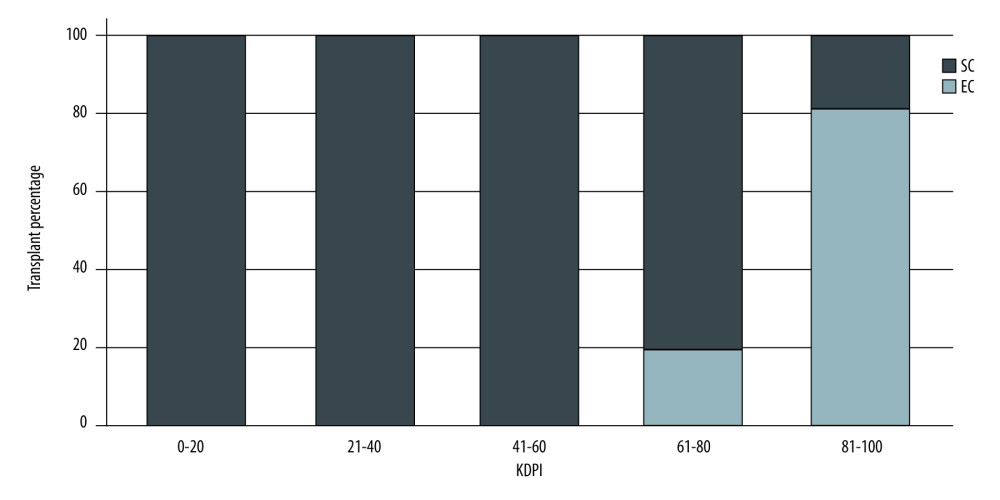 Figure 2. Donor classification according to KDPI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 2. Donor classification according to KDPI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. 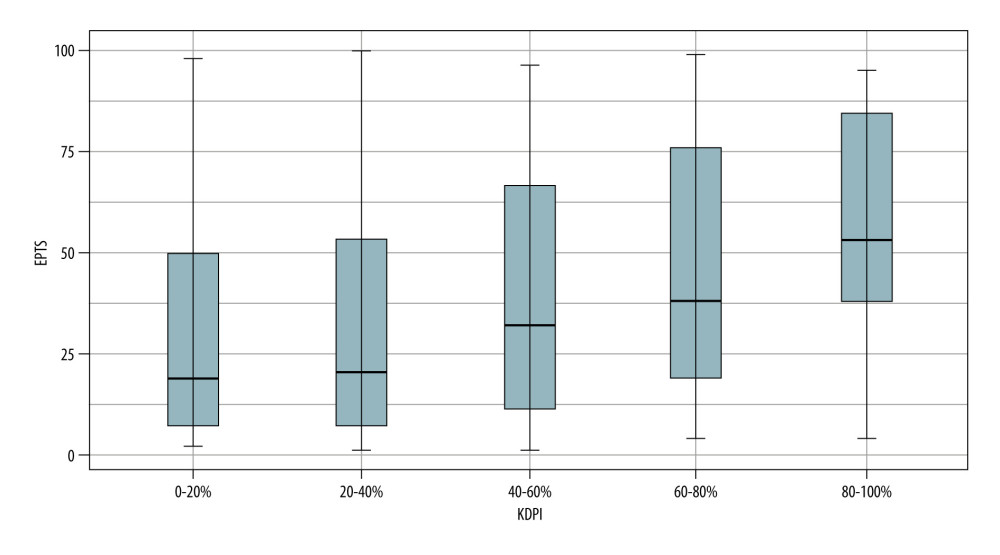 Figure 3. KDPI values according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 3. KDPI values according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. 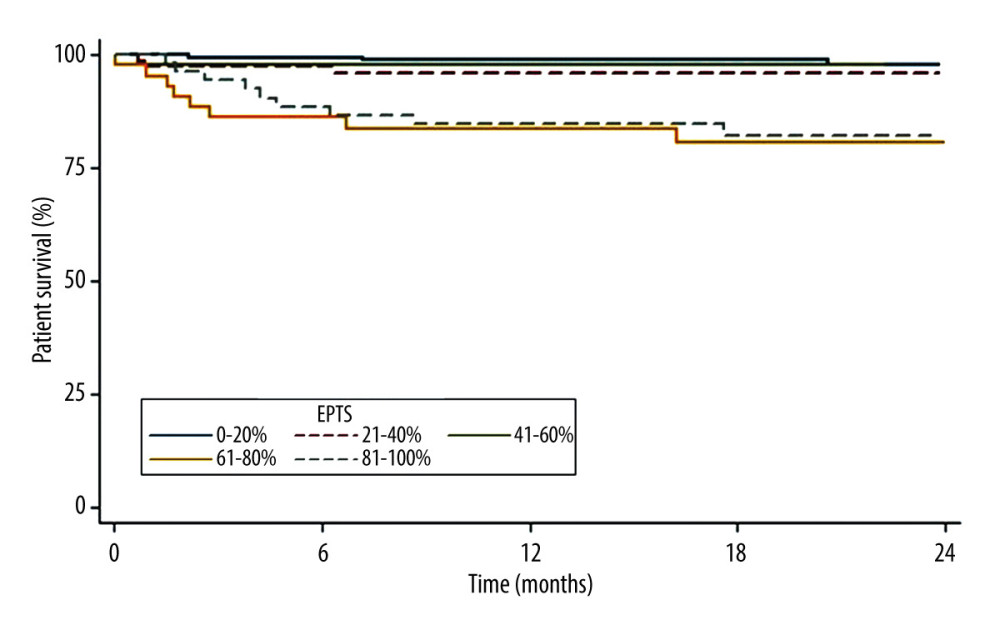 Figure 4. Patient survival according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 4. Patient survival according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. 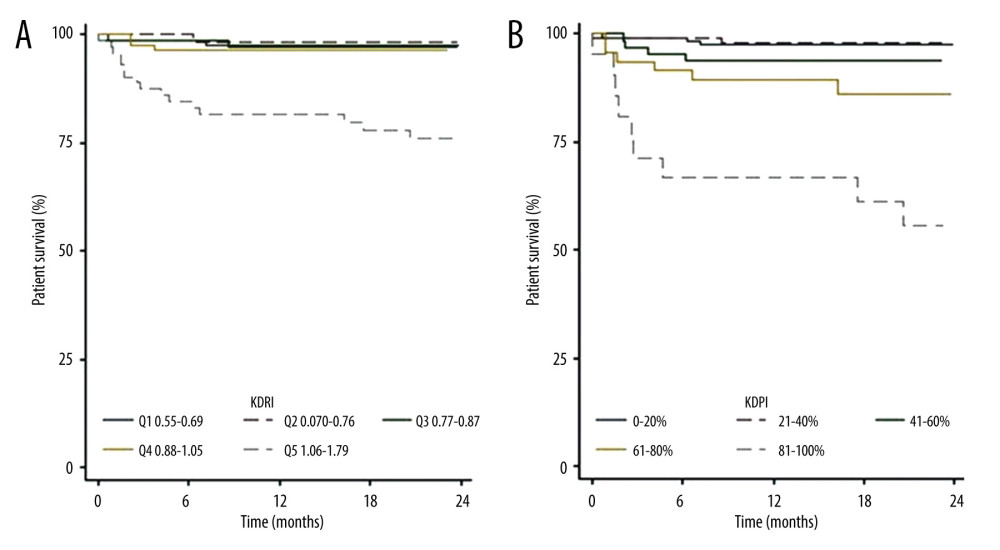 Figure 5. (A) Patient survival according to KDRI. (B) Patient survival according to KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 5. (A) Patient survival according to KDRI. (B) Patient survival according to KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. 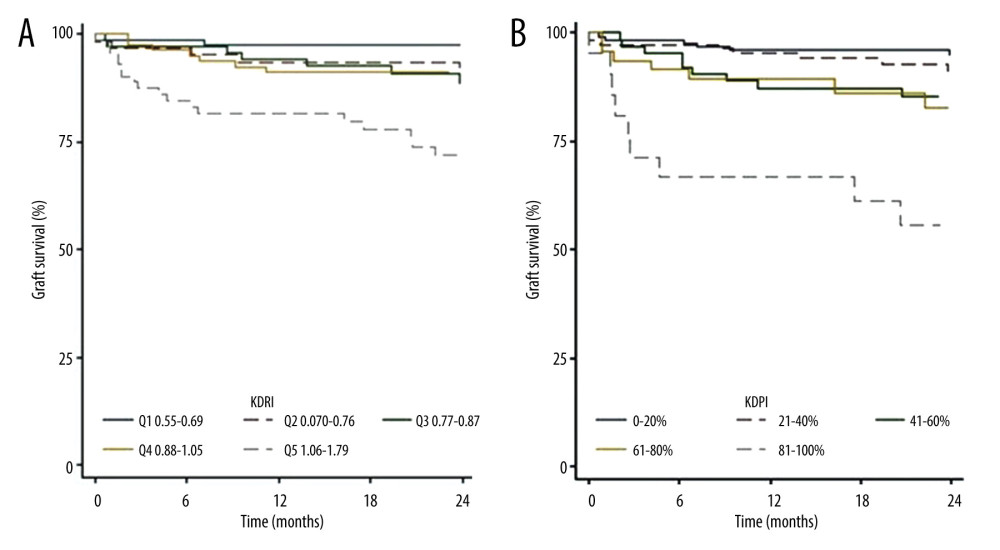 Figure 6. (A) Graft survival according to the KDRI. (B) Graft survival according to the KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 6. (A) Graft survival according to the KDRI. (B) Graft survival according to the KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. 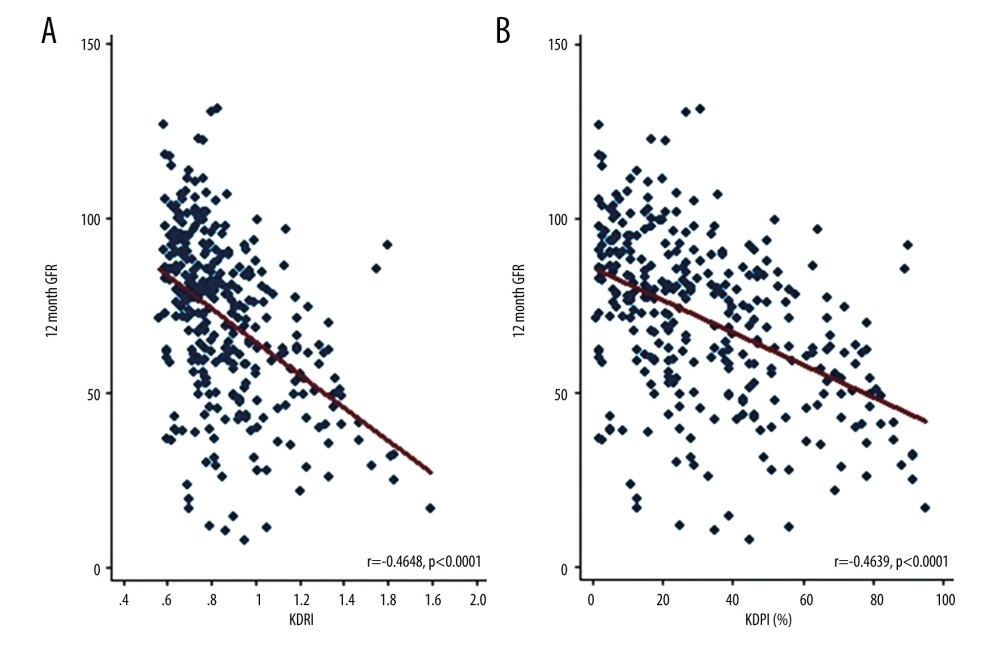 Figure 7. (A) Correlation between glomerular filtration rate at 12 months post-transplantation and KDRI values. (B) Correlation between glomerular filtration rate at 12 months post-transplantation and KDPI values. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 7. (A) Correlation between glomerular filtration rate at 12 months post-transplantation and KDRI values. (B) Correlation between glomerular filtration rate at 12 months post-transplantation and KDPI values. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Tables
Table 1. Demographic and clinical characteristics of kidney transplant recipients at 2 transplant centers in Cali, Colombia, between 2010 and 2017.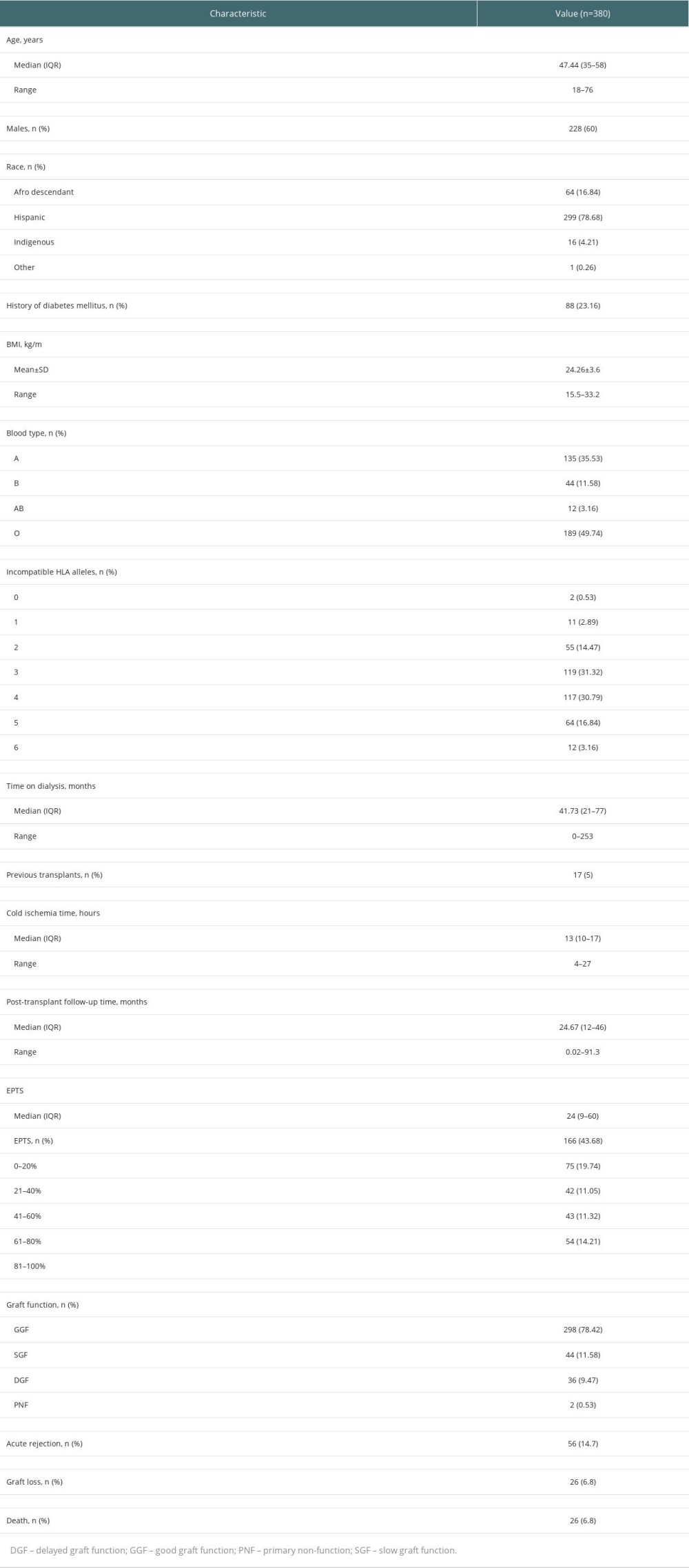 Table 2. Demographic and clinical characteristics of deceased kidney transplant donors at 2 transplant centers in Cali, Colombia, between 2010 and 2017.
Table 2. Demographic and clinical characteristics of deceased kidney transplant donors at 2 transplant centers in Cali, Colombia, between 2010 and 2017.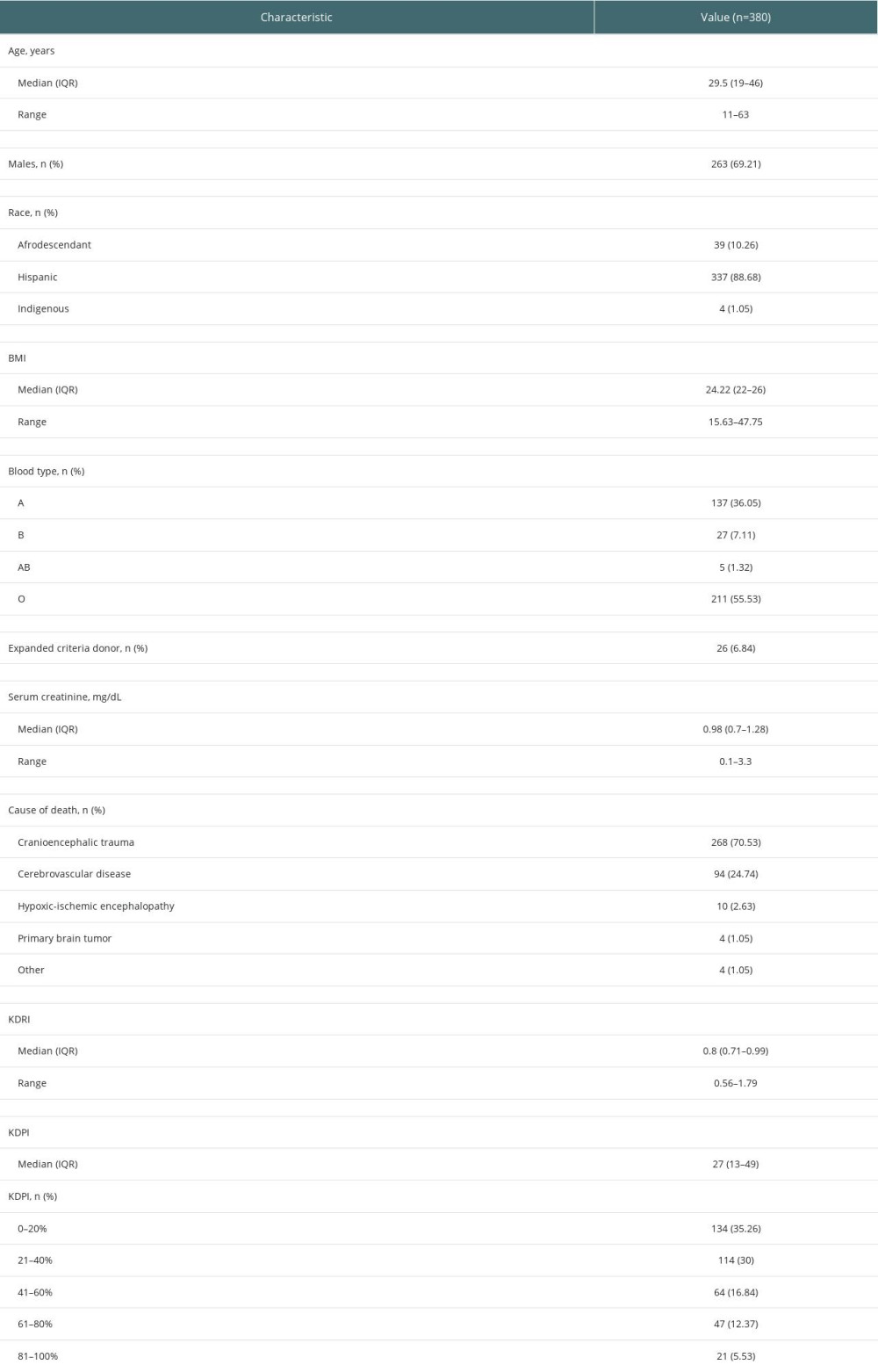
References
1. Port FK, Bragg-Gresham JL, Metzger RA, Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors: Transplantation, 2002; 74(9); 1281-86
2. Rao PS, Schaubel DE, Guidinger MK, A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index: Transplantation, 2009; 88(2); 231-36
3. Massie AB, Luo X, Chow EK, Survival benefit of primary deceased donor transplantation with high-KDPI kidneys: Am J Transplant, 2014; 14(10); 2310-16
4. Peters-Sengers H, Heemskerk MBA, Geskus RB, Validation of the Prognostic Kidney Donor Risk Index Scoring System of deceased donors for renal transplantation in the Netherlands: Transplantation, 2018; 102(1); 162-70
5. Gourishankar S, Grebe SO, Mueller TF, Prediction of kidney graft failure using clinical scoring tools: Clin Transplant, 2013; 27(4); 517-22
6. Han M, Jeong JC, Koo TY, Kidney donor risk index is a good prognostic tool for graft outcomes in deceased donor kidney transplantation with short, cold ischemic time: Clin Transplant, 2014; 28(3); 337-44
7. OPTN: A guide to calculating and interpreting the Estimated Post-Transplant Survival (EPTS) score used in the Kidney Allocation System (KAS) what is the EPTS score?, 2019; 1-9
8. OPTN: Organ Transplantation Network: Allocation calculators, n.d Retrieved October 31, 2018https://optn.transplant.hrsa.gov/data/allocation-calculators/
9. OPTN, OPTN Policy Update 12-2014: Kidney Allocation System Language, 2014; 1-22.papers3
10. El-Zoghby ZM, Stegall MD, Lager DJ, Identifying specific causes of kidney allograft loss: Am J Transplant, 2009; 9(3); 527-35
11. Humar A, Johnson EM, Payne WD, Effect of initial slow graft function on renal allograft rejection and survival: Clin Transplant, 1997; 11(6); 623-27
12. Wang CJ, Wetmore JB, Israni AK, Old versus new: Progress in reaching the goals of the new kidney allocation system: Hum Immunol, 2017; 78(1); 9-15
13. Chopra B, Sureshkumar KK, Changing organ allocation policy for kidney transplantation in the United States: World J Transplant, 2015; 5(2); 38-43
14. Ramanathan R, Gupta G, Kim J, Retroactive application of the new kidney allocation system to renal transplants performed in the ECD/SCD era: Clin Transplant, 2015; 29(12); 1148-55
15. Hart A, Smith JM, Skeans MA, OPTN/SRTR 2015 annual data report: Kidney: Am J Transplant, 2017; 17(Suppl 1); 21-116
16. Israni AK, Salkowski N, Gustafson S, New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes: J Am Soc Nephrol, 2014; 25(8); 1842-48
17. Jay CL, Washburn K, Dean PG, Survival benefit in older patients associated with earlier transplant with high KDPI kidneys: Transplantation, 2017; 101(4); 867-72
18. Clayton PA, McDonald SP, Snyder JJ, External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States: Am J Transplant, 2014; 14(8); 1922-26
19. Del Moral Martín RMG, Retamero Díaz JA, Cava Molina M, Validation of KDRI/KDPI for the selection of expanded criteria kidney donors: Nefrologia, 2018; 38(3); 297-303
20. Lehner LJ, Kleinsteuber A, Halleck F, Assessment of the Kidney Donor Profile Index in a European cohort: Nephrol Dial Transplant, 2018; 33(8); 1465-72
21. Hart A, Gustafson SK, Skeans MA, OPTN/SRTR 2015 Annual Data Report: Early effects of the new kidney allocation system: Am J Transplant, 2017; 17(Suppl 1); 543-64
22. Zens TJ, Danobeitia JS, Leverson G, The impact of kidney donor profile index on delayed graft function and transplant outcomes: A single-center analysis: Clin Transplant, 2018; 32(3); e13190
23. Philipse E, Lee APK, Bracke B, Does Kidney Donor Risk Index implementation lead to the transplantation of more and higher-quality donor kidneys?: Nephrol Dial Transplant, 2017; 32(11); 1934-38
24. Bae S, Massie AB, Luo X, Changes in discard rate after the introduction of the Kidney Donor Profile Index (KDPI): Am J Transplant, 2016; 16(7); 2202-7
25. Ekser B, Powelson JA, Fridell JA, Is the kidney donor profile index (KDPI) universal or UNOS-specific?: Am J Transplant, 2017; 18(4); 1031-32
26. Pascual J, Pérez-Sáez MJ, Kidney Donor Profile Index: Can it be extrapolated to our enviroment?: Nefrologia, 2016; 36(5); 465-68
27. Lee AP, Abramowicz D, Is the Kidney Donor Risk Index a step forward in the assessment of deceased donor kidney quality?: Nephrol Dial Transplant, 2015; 30(8); 1285-90
28. Wohlfahrtova M, Viklicky O, New strategies for evaluating the quality of kidney grafts from elderly donors: Transplant Rev (Orlando), 2015; 29(4); 212-18
29. Heilman RL, Green EP, Reddy KS, Potential impact of risk and loss aversion on the process of accepting kidneys for transplantation: Transplantation, 2017; 101(7); 1514-17
Figures
 Figure 1. Donor classification according to KDRI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 1. Donor classification according to KDRI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Figure 2. Donor classification according to KDPI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 2. Donor classification according to KDPI category. SC – standard criteria; EC – expanded criteria. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Figure 3. KDPI values according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 3. KDPI values according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Figure 4. Patient survival according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 4. Patient survival according to EPTS index. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Figure 5. (A) Patient survival according to KDRI. (B) Patient survival according to KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 5. (A) Patient survival according to KDRI. (B) Patient survival according to KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Figure 6. (A) Graft survival according to the KDRI. (B) Graft survival according to the KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 6. (A) Graft survival according to the KDRI. (B) Graft survival according to the KDPI. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Figure 7. (A) Correlation between glomerular filtration rate at 12 months post-transplantation and KDRI values. (B) Correlation between glomerular filtration rate at 12 months post-transplantation and KDPI values. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press.
Figure 7. (A) Correlation between glomerular filtration rate at 12 months post-transplantation and KDRI values. (B) Correlation between glomerular filtration rate at 12 months post-transplantation and KDPI values. StataCorp. 2017. Stata 15 Base Reference Manual. College Station, TX: Stata Press. Tables
 Table 1. Demographic and clinical characteristics of kidney transplant recipients at 2 transplant centers in Cali, Colombia, between 2010 and 2017.
Table 1. Demographic and clinical characteristics of kidney transplant recipients at 2 transplant centers in Cali, Colombia, between 2010 and 2017. Table 2. Demographic and clinical characteristics of deceased kidney transplant donors at 2 transplant centers in Cali, Colombia, between 2010 and 2017.
Table 2. Demographic and clinical characteristics of deceased kidney transplant donors at 2 transplant centers in Cali, Colombia, between 2010 and 2017. Table 1. Demographic and clinical characteristics of kidney transplant recipients at 2 transplant centers in Cali, Colombia, between 2010 and 2017.
Table 1. Demographic and clinical characteristics of kidney transplant recipients at 2 transplant centers in Cali, Colombia, between 2010 and 2017. Table 2. Demographic and clinical characteristics of deceased kidney transplant donors at 2 transplant centers in Cali, Colombia, between 2010 and 2017.
Table 2. Demographic and clinical characteristics of deceased kidney transplant donors at 2 transplant centers in Cali, Colombia, between 2010 and 2017. In Press
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
02 Apr 2024 : Original article
Effect of Dexmedetomidine Combined with Remifentanil on Emergence Agitation During Awakening from Sevoflura...Ann Transplant In Press; DOI: 10.12659/AOT.943281
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








