Abstract
Background
Targeted approaches such as targeted axillary dissection (TAD) or sentinel lymph node biopsy (SLNB) showed false-negative rates of < 10% compared with axillary lymph node dissection (ALND) in patients with nodal-positive breast cancer undergoing neoadjuvant systemic treatment (NAST). We aimed to evaluate real-world oncologic outcomes for different axillary staging techniques.
Methods
We identified nodal-positive breast cancer patients undergoing NAST from 2016 to 2021 from the state cancer registry of Baden-Wuerttemberg, Germany. Invasive disease-free survival (iDFS) was assessed using Kaplan–Meier statistics and multivariate Cox regression models (adjusted for age, ypN stage, ypT stage, and tumor biologic subtype).
Results
A total of 2698 patients with a median follow-up of 24.7 months were identified: 2204 underwent ALND, 460 underwent SLNB (255 with ≥ 3 sentinel lymph nodes [SLNs] removed, 205 with 1–2 SLNs removed), and 34 underwent TAD. iDFS 3 years after surgery was 69.7% (ALND), 76.6% (SLNB with ≥ 3 SLNs removed), 76.7% (SLNB with < 3 SLNs removed), and 78.7% (TAD). Multivariate Cox regression analysis showed no significant influence of different axillary staging techniques on iDFS (hazard ratio [HR] for SLNB with < 3 SLNs removed 0.96, 95% confidence interval [CI] 0.62–1.50; HR for SLNB with ≥ 3 SLNs removed 0.86, 95% CI 0.56–1.3; HR for TAD 0.23, 95% CI 0.03–1.64; ALND reference), and for ypN+ (HR 1.92, 95% CI 1.49–2.49), triple-negative breast cancer (HR 2.35, 95% CI 1.80–3.06), and ypT3-4 (HR 2.93, 95% CI 2.02–4.24).
Conclusion
These real-world data provide evidence that patient selection for de-escalated axillary surgery for patients with nodal-positive breast cancer undergoing NAST was successfully adopted and no early alarm signals of iDFS detriment were detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Therapeutic breast cancer management has undergone several paradigm shifts over the past decades. Radical surgery, including mastectomy and axillary lymph node dissection (ALND), used to be the standard of care during the Halstedian paradigm. With the introduction of modern, multimodality treatment (surgery, systemic treatment, radiotherapy), more tailored and less invasive surgical interventions such as breast-conserving surgery and sentinel lymph node biopsy (SLNB) were established. These methods are based on clinical trials that demonstrate non-inferior oncologic outcomes.1,2,3,4 Breast-conserving surgery and SLNB were initially established in the adjuvant setting and also subsequently in the neoadjuvant setting.
However, an important knowledge gap remains in the optimal axillary staging technique for patients with nodal-positive breast cancer undergoing neoadjuvant systemic treatment (NAST). Several diagnostic studies compared the diagnostic performance of SLNB with ALND and demonstrated that SLNB has a high risk of leaving tumor behind (false-negative rate > 10%).5,6,7 More recently, targeted approaches such as targeted axillary dissection (TAD) or SLNB with ≥ 3 sentinel lymph nodes (SLNs) removed showed missed cancer rates of < 10% compared with ALND.7,8,9 Based on these diagnostic results, international guidelines have permitted the use of such targeted axillary staging techniques instead of ALND;10,11 however, to date, there is no evidence on actual oncologic outcomes for de-escalated axillary staging following NAST.
In this study, we evaluated oncologic outcomes for different axillary staging techniques in nodal-positive breast cancer patients undergoing NAST. We utilized data reported to the cancer register of Baden-Wuerttemberg and thus represented routine clinical practice in a real-world patient population.
Methods
Patient Selection
Patient records were obtained from the clinical Cancer Registry Database (Klinisches Landeskrebsregister [KLR]) of the German Federal State Baden-Wuerttemberg (BW), with a population of over 11 million in 2019. The KLR BW collects standardized clinical, diagnostic, treatment, and follow-up information for all patients who are diagnosed with cancer in the BW state. The standardized data transfer from the treating cancer centers to the cancer registry includes patient age, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 neu receptor status, grading, tumor biology, cTNM stage, pTNM stage, lymph nodes removed, lymph nodes with cancer, type of breast surgery (mastectomy vs. breast-conserving), and type of axillary staging (SLNB vs. ALND) and follow-up (recurrences, remission, death, etc.).
Patient Selection
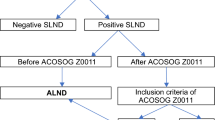
The inclusion criteria for selecting patients were (1) malignant neoplasm of the breast (International Classification of Diseases, Tenth Revision [ICD-10] code C50) diagnosed between 2016 and 2021; (2) TNM clinical classification of the lymph node and metastases were cN+ and cM0, respectively; (3) reported neoadjuvant therapy before the first surgery; (4) standardized surgical procedure codes (operation and procedure [OPS] codes) of first surgery included either an ALND or SLNB code (i.e. OPS code is either from 5-401, 5-402, 5-404, 5-406, 5-407); and (5) follow-up was available for at least 1 year. The timeframe was chosen with regard to the introduction of TAD in 20168 and at least 1-year of follow-up data. Patients with metastatic disease were not included in the analysis. All selection procedure steps are illustrated in Fig. 1.
To collect information regarding the diagnosis and therapy, the standardized database records, as well as pathology reports, were considered. Information retrieved from the database included patient age, ER and PR status, HER2 neu receptor status, grading, tumor biology, cTNM stage, pTNM stage, and type of (axillary) surgery (OPS code). Due to its novelty, TAD is not yet part of the standardized data capturing and therefore was identified based on the pathology report text (see below). Pathology reports of the patients selected by diagnosis year, ICD-10, and OPS code were automatically scanned using an in-house text analysis program (overall, 22,805 pathology reports). The number of removed lymph nodes, pTNM code, and the references to NAST, sentinel lymph node dissection, or marking were searched, extracted, and analyzed in addition to the available records in the database. Cases with unknown or ambiguous information in any of these variables were excluded.
The patient groups were built using the following multistep procedure:
-
(1)
First, patients who had ALND as the first OPS code (OPS codes 5-402, 5-404, 5-406, 5-407) or those who recorded SLNB as the first OPS code (OPS code 5-401), but in whom the number of lymph nodes removed was above 10, were assigned to the ALND group.
-
(2)
We then identified patients with a first OPS code of 5-401 (excision of single lymph nodes) and < 11 SLNs removed, and with available full-text pathology reports (for 339 patients in total), which allowed for text-based analysis as to whether TAD was performed (removal of the clipped lymph node plus SLNB).
-
(3)
Preliminary selection by text mining; 171 pathology reports were then manually inspected, and the TAD group was extracted based on analysis of the pathology reports (removal of the clipped lymph node plus SLNB).
-
(4)
The remaining patients with a first OPS code of 5-401 and < 11 SLNs removed (including those without a pathology report available) were assigned to either the SLNB with < 3 SLNs removed group or the SLNB with ≥ 3 SLNs removed group.
Statistical Analysis
Invasive disease-free survival (iDFS) was assessed using Kaplan–Meier statistics and multivariate Cox regression models (adjusted for age, ypN stage, ypT stage, and tumor biologic subtype). Time to event was defined as the time from surgery to an event, and events were defined as either local recurrence, distant recurrence, or death. Univariate and multivariate Cox proportional hazard models were used to evaluate the prognostic value of the different axillary staging techniques on the risk of experiencing an event. Hazard ratios (HRs) per unit score are reported. Kaplan–Meier estimators were used to obtain the iDFS rates after 2 and 4 years of follow-up. Time to event was compared using log-rank tests.
Ethics
Ethical review and approval were waived for this study due to the de-identified information of patients included in the BW database.
Results
Flow of Participants
A total of 3307 patients with cN+, cM0 breast cancer undergoing NAST and axillary surgery from 2016 to 2021 were included, of whom 2971 had at least 1 year of follow-up and 2698 had available information regarding axillary staging techniques (see also Fig. 1).
Patient Demographic and Clinical Information
Table 1 illustrates patient demographic and clinical information. Among the 2698 patients analyzed, median patient age was 59.6 years (standard deviation [SD] 14.4). With respect to tumor stage, 36.8% (605/1643) accounted for pT0 stage and 50.8% (1121/2205) accounted for pN0 stage. Tumor biology was hormone receptor (HR)-positive/HER2-negative in 40.2% (1043/2594) of cases, HR-positive/HER2-positive in 19.8% (513/2594), HR-negative/HER2-positive in 10.3% (266/2594), triple-negative in 16.7% (433/2594), and changing receptor status in 13.1% (339/2594). Axillary staging technique was ALND in 81.7% (2204/2698) of patients, SLNB with ≥ 3 SLNs removed in 9.5% (255/2698), SLNB with < 3 SLNs removed in 7.6% (205/2698), and TAD in 1.3% (34/2698). Distribution of baseline clinical and patient characteristics for the different axillary staging techniques is also listed in Table 1. There were significant differences for c/ypT stage, c/ypN stage, tumor biologic subtype, type of neoadjuvant treatment, and adjuvant radiotherapy among the different axillary staging groups, with higher tumor stages in the ALND group. Notably, there were no significant differences for irradiation volumes among the ALND patients (53.6, SD 6.7), SLNB patients with ≥ 3 SLNs removed (55.3, SD 5.9), SLNB patients with < 3 SLNs removed (53.8, 6.1), and TAD patients (50.4, SD 10.0).
Oncologic Outcomes for Different Axillary Staging Techniques
Median follow-up was 24.7 months. Kaplan–Meier plots of iDFS for the different axillary staging techniques (ALND, SLNB, and TAD) in the whole cohort and stratified for ypN+ versus ypN0, ypT+ versus ypT0, and tumor biologic subtype are illustrated in Figs. 2 and 3. In the overall cohort, significant differences in iDFS between the ALND, SLNB, and TAD groups were observed (log rank p = 0.01): iDFS 3 years after surgery was 69.7% (ALND), 76.6% (SLNB with ≥ 3 SLNs removed), 76.7% (SLNB with < 3 SLNs removed), and 78.7% (TAD).
Kaplan–Meier plots of invasive disease-free survival for different axillary staging techniques. (a) HR-positive/HER2-negative; (b) HR-positive/HER2-positive; (c) HR-negative/HER2-positive; (d) TNBC; (e) changing receptor status. ALND axillary lymph node dissection, HR hormone receptor, HER2 human epidermal growth factor receptor 2, SLNB sentinel lymph node biopsy, TAD targeted axillary dissection, TNBC triple-negative breast cancer
Multivariate Cox regression analysis, including axillary staging technique (without TAD due to the low sample size), age, pTNM stage, and tumor biologic subtype (Fig. 4), showed no significant influence of different axillary staging techniques on iDFS: HR 0.86 (95% confidence interval [CI] 0.56–1.31) for SLNB with ≥ 3 SLNs removed; HR 0.97 (95% CI 0.62–1.51) for SLNB with <3 SLNs removed; ALND (reference). iDFS was significantly influenced by ypN+ status (HR 1.92, 95% CI 1.49–2.49, p < 0.001, compared with ypN0), ypT+ status (HR 1.61, 95% CI 1.21–2.15, p < 0.001 for ypT1-2; and HR 2.96, 95% CI 2.04–4.29, p < 0.001 for ypT3-4, compared with ypT0), and receptor status (HR 2.35, 95% CI 1.80–3.07, p < 0.001 for triple-negative breast cancer (TNBC); and HR 2.22, 95% CI 1.70–2.91, p < 0.001 for changing receptor status, compared with HR-positive/HER2-negative). Also in the subgroup of patients with ypN0 status (Fig. 4b), no significant influence of different axillary staging techniques on iDFS was observed: HR 0.91 (95% CI 0.53–1.60) for SLNB with ≥3 SLNs removed; HR 1.21 (95% CI 0.72–2.00) for SLNB with <3 SLNs removed; ALND (reference).
Multivariate Cox regression analysis without the TAD group. (a) Whole cohort; (b) ypN0 subgroup. AIC Akaike information criterion, ALND axillary lymph node dissection, HRn hormone receptor-negative, HRp hormone receptor-positive, HER2n human epidermal growth factor receptor 2-negative, HER2p human epidermal growth factor receptor 2-positive, SLNB sentinel lymph node biopsy, TAD targeted axillary dissection, TNBC triple-negative breast cancer
If TAD was included in the multivariate Cox regression analysis, it resulted in an HR of 0.23 (95% CI 0.032–1.64), which should however be interpreted with caution considering the low sample size (Supplemental Fig. 1).
Subgroup Analysis
When stratified for ypN and ypT status (Fig. 2) as well as receptor status (Fig. 3), differences in iDFS between the different axillary staging techniques were descriptively larger in patients with ypN+ versus ypN0 disease (Fig. 2b, c), in patients with ypT+ versus ypT0 disease (Fig. 2d, e), and in patients with TNBC (Fig. 3d).
Figure 5 illustrates the fractions of patients with ypN0 versus ypN+ and ypT0 versus ypT+ status for the different axillary staging techniques in patients were both pieces of information were available (n = 1612): The proportion of patients with pN0 status was significantly lower in the ALND group compared with the SLNB with ≥ 3 SLNs removed, SLNB with < 3 SLNs removed, and TAD groups (46.3% vs. 66.2%, 74.7%, and 69.5%, respectively).
Fraction of patients with residual disease after surgery. Groups A, L, S, and T denote the ALND, SLNB with < 3 SLNs removed, SLNB with ≥3 SLNs removed, and TAD groups, respectively. The bootstrapping procedure was used for computation of mean and standard deviation values in each group. ALND axillary lymph node dissection, SLNB sentinel lymph node biopsy
Discussion
In this study, we evaluated oncologic outcomes for different axillary staging techniques in nodal-positive breast cancer patients undergoing NAST, specifically for ALND, SLNB (≥ 3 and < 3 SLNs removed), and TAD. After a median follow-up of 24.7 months, multivariate Cox regression analysis showed no significant influence of different axillary staging techniques on iDFS in the overall cohort (Fig. 4a). Also in the subgroup of ypN0 patients, where de-escalated axillary surgery is known to occasionally miss residual cancer, no significant influence of different axillary staging techniques on iDFS was observed (Fig. 4b). These data provide evidence that patient selection for de-escalated axillary surgery (in combination with radiotherapy) was successfully adopted in a real-world population sample and no early alarm signals of survival detriment were detected.
We have learned in the past decades that most breast cancer patients can be spared radical surgery in times of modern multimodality treatment. At first, clinical trials demonstrated equivalent survival for breast-conserving therapy (BCT, breast-conserving surgery and radiotherapy) compared with mastectomy in the early 2000s.10,11 Later, clinical trials demonstrated equivalent survival of SLNB compared with ALND in the adjuvant setting for cN0 patients, despite leaving some tumor behind (about 10% of missed lymph node metastasis for SLNB compared with ALND).12,13 From these trials, the assumption arose that a false-negative rate of 10% would not translate into impaired oncologic outcomes. Consequently, as subsequent trials showed false-negative rates of >10% for SLNB in cN+ patients undergoing NAST, ALND remained the standard of care for these patients. Recent improvements in less invasive targeted axillary staging techniques showed improved false-negative rates compared with ALND: about 2% for TAD (removal of clipped node plus SLNB)8,9,14 and about 8% for SLNB with ≥ 3 SLNs removed.14 Notably, based on these diagnostic findings, international guidelines now allow the use of such targeted axillary staging for cN+ patients undergoing NAST despite missing survival data. Another recent study evaluated 3-year oncologic outcomes of 199 patients undergoing TAD (n = 119) versus TAD with ALND (80). After 3 years of follow-up, iDFS was 82.4% (95% CI 71.5–89.4) in the TAD + ALND group, and 91.2% (95% CI 84.2–95.1) in the TAD group (p = 0.04); axillary recurrence occurred in 1.4% (95% CI 0–54.8) and 1.8% (95% CI 0–36.4), respectively (p = 0.56). TAD was not associated with an increased risk of recurrence (HR 0.83, 95% CI 0.34–2.05; p = 0.69) or death (HR 1.07, 95% CI 0.31–3.70; p = 0.91) in the adjusted multivariate Cox regression.15 Our analyses provides additional information, especially with respect to the clinically relevant subgroup of patients with ypN0 disease, where SLNB or TAD is known to occasionally miss residual disease (false negative rate, see above). Also in the subgroup of patients with ypN0 status (Fig. 4b), no significant influence of different axillary staging techniques on iDFS was observed, with an HR of 0.91 (95% CI 0.53–1.60) for SLNB with ≥ 3 SLNs removed and an HR of 1.21 (95% CI 0.72–2.00) for SLNB with < 3 SLNs removed. Notably, our registry analysis also provides insights into irradiation practices in case of de-escalated surgical axillary staging: no significant differences were observed for irradiation volumes among the ALND (53.6, SD 6.7), SLNB with ≥ 3 SLNs removed (55.3, SD 5.9), SLNB with < 3 SLNs removed (53.8, 6.1), and TAD (50.4, SD 10.0) groups. Although we acknowledge the small sample size in some groups, there does not seem to be a trend for more irradiation in the case of de-escalated surgical staging. Thus, the results of our analysis provide additional information regarding the oncologic outcomes for SLNB, and add to the available survival outcomes for different axillary staging techniques in nodal-positive breast cancer patients undergoing NAST.
Tailored oncologic breast and axillary surgery may not only result in equivalent survival but may actually improve survival. A recent meta-analysis comparing BCT with mastectomy in times of modern multimodality (14 studies from 1980 to 2014, n = 19,819) suggests that BCT is actually associated with improved oncologic outcomes: all-cause mortality in favor of BCT (HR 0.78, 95% CI 0.69–0.89; p < 0.001), locoregional recurrence in favor of BCT (HR 0.64, 95% CI 0.48–0.85; p = 0.002), and distant recurrence in favor of BCT (HR 0.70, 95% CI 0.530.94; p = 0.02).3 Mechanisms of action include reduction of rare but severe surgical complications such as thrombosis and sepsis. In our study, significant differences in iDFS between the ALND, SLNB, and TAD groups were observed in favor of the targeted approaches (log rank p = 0.01), which may however reflect differences in tumor stages among the axillary staging groups. For example, the proportion of patients with ypN+ stage within these four groups was 53.5%, 33.8%, 25.2%, and 30.4%, respectively (p < 0.001). When adjusting for ypT/ ypN stages, age, and tumor biologic subtype, no significant influence on iDFS for the different axillary staging techniques was observed. Further evaluation in larger (registry) trials with longer follow-up seems warranted.
The interactions between axillary staging and adjuvant systemic therapy are an emerging topic of high relevance. For example, pN status is frequently used to identify high-risk patients eligible for escalated adjuvant treatment (abemaciclib for HR-positive/HER2-negative patients with pN2 status or pN1 status with additional risk factors, trastuzumab emtansine (T-DM1) for HER2-positive patients with residual disease, and capecitabine for TNBC patients with residual disease).16,17,18 It should be noted that the differences in distribution of pN status between different axillary staging techniques observed in our study may not be completely related to the actual disease stage. A higher proportion of patients with pN+ status in the ALND group may also reflect the fact that we actually leave some tumor behind in the axilla when using targeted techniques so that the proportion of patients with pN+ stage is systematically lower within these populations. Indeed, the American College of Surgeons (ACOSOG) Z0011 trial showed that almost 14% of patients who underwent SLNB with 1–2 positive SLNs had ≥4 positive nodes after undergoing subsequent ALND.4 Thus, future research may develop new risk scores to identify patients with (high) nodal disease burden for accurate indications of escalated adjuvant systemic treatment in times of de-escalated axillary surgery.
A critical discussion focuses around the role of clinical lymph node assessment after NAST. Current national guidelines allow the use of targeted axillary staging techniques (SLNB, TAD) for patients with nodal-positive disease before NAST (cN+) who convert to nodal-negative disease (ycN0) on imaging, whereas patients who remain nodal positive (ycN+) on imaging are recommended to undergo ALND.10,11 In the present analysis, patients with cN+ disease were included but ycN status is unknown as this information is not routinely collected by the cancer registry. There is however an ongoing discussion about the usefulness of axillary response assessment by imaging after NAST. Axillary ultrasound, which is most commonly used to assess response of the axillary lymph nodes, shows limited diagnostic accuracy to determine the ycN status; among patients with negative nodes on ultrasound after NAST (ycN0), about 50% present with residual axillary disease in the surgical specimen.19 Taking ycN0 status as a prerequisite for targeted axillary staging techniques may thus be inadequate and ineffective. Future research may focus on this area and provide more in-depth comparisons of oncologic outcomes for patients undergoing targeted axillary staging with ycN+ status.
Some limitations of the present analysis must be kept in mind. First, the sample size of the TAD group was small, which is why this group was not included in the multivariate Cox regression analysis. Moreover, a median follow-up of 24.7 months may enable first conclusions with respect to local recurrence events, but does not suffice to conclude safe long-term overall or distant DFS. Future studies with longer-term follow-up are welcomed to fully inform this discussion. Second, as TAD is relatively new, it is not routinely captured by the cancer registry. We identified patients with available full-text pathology reports, which allowed for text-based analysis as to whether TAD was performed (removal of clipped lymph node plus SLNB); however, this approach may have led to selection bias).
Conclusion
These real-world data provide evidence that patient selection for de-escalated axillary surgery (such as TAD or SLNB instead of ALND) in combination with radiotherapy for patients with nodal-positive breast cancer undergoing NAST was successfully adopted in a real-world population sample and no early alarm signals of iDFS detriment were detected. Future studies with longer-term follow-up are encouraged to fully inform this discussion.
Data Availability
Will individual participant data be available (including data dictionaries)?: No. What data in particular will be shared?: No. What other documents will be available?: Study protocol. When will data be available (start and end dates)?: No. With whom?: No. For what types of analyses?: No. By what mechanism will data be made available?: No
References
Early Breast Cancer Trialists’ Collaborative Group. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39.
Mieog JSD, Van Der Hage JA, Van De Velde CJH. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–200.
Fancellu A, Houssami N, Sanna V, Porcu A, Ninniri C, Marinovich ML. Outcomes after breast-conserving surgery or mastectomy in patients with triple-negative breast cancer: meta-analysis. Br J Surg. 2021;108:760–8.
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection versus no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918–26.
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18.
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA. 2013;310:1455–61.
Shirzadi A, Mahmoodzadeh H, Qorbani M. Assessment of sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer in two subgroups: initially node negative and node positive converted to node negative: a systemic review and meta-analysis. J Res Med Sci. 2020;24:18.
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patientswith node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–8.
Kuemmel S, Heil J, Rueland A, Seiberling C, Harrach H, Schindowski D, et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2022;276(5):e553–62.
Giordano SH, Elias AD, Gradishar WJ. NCCN guidelines updates: breast cancer. J Natl Compr Canc Netw. 2018;16:605–10.
Wöckel A, Festl J, Stüber T, Brust K, Stangl S, Heuschmann PU, et al. Interdisciplinary Screening, diagnosis, therapy and follow-up of breast cancer. Guideline of the DGGG and the DKG (S3-level, AWMF registry number 032/045OL, December 2017): Part 1 with recommendations for the screening, diagnosis and therapy of breast Ca. Geburtshilfe Frauenheilkd. 2018;78:927–48.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33.
Simons JM, van Nijnatten TJA, van der Pol CC, Luiten EJT, Koppert LB, Smidt ML. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2019;269(3):432–42.
Kuemmel S, Heil J, Bruzas S, Breit E, Schindowski D, Harrach H, et al. Safety of targeted axillary dissection after neoadjuvant therapy in patients with node-positive breast cancer. JAMA Surg. 2023;158:807–15.
Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77–90.
von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59.
Morency D, Dumitra S, Parvez E, Martel K, Basik M, Robidoux A, et al. Axillary lymph node ultrasound following neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: results from the SN FNAC study. Ann Surg Oncol. 2019;26:4337–45.
Acknowledgment
André Pfob’s work is supported by the Digital Oncology Fellowship provided by the German Cancer Research Center (DKFZ) and the National Center for Tumor Diseases (NCT) Heidelberg.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AP, DBK, IS, FR, PM, and JH contributed to the data collection, and reviewed and approved the final manuscript draft. The manuscript was drafted by AP, IS, and DK.
Corresponding authors
Ethics declarations
DISCLOSURE
André Pfob, Daria B. Kokh, Irina Surovtsova, Fabian Riedel, Philipp Morakis, and Joerg Heil have no conflicts of interest to declare in relation to this work.
Ehtics Approval and Consent to Participate
This study was performed in line with the principles of the Declaration of Helsinki. Ethical review and approval were waived for this study due to the de-identified information of the patients included in the BW database.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An abstract reporting the final results was presented at the American Society of Clinical Oncology Annual Meeting, 2–6 June 2023 (#412896).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pfob, A., Kokh, D.B., Surovtsova, I. et al. Oncologic Outcomes for Different Axillary Staging Techniques in Patients with Nodal-Positive Breast Cancer Undergoing Neoadjuvant Systematic Treatment: A Cancer Registry Study. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15292-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15292-y